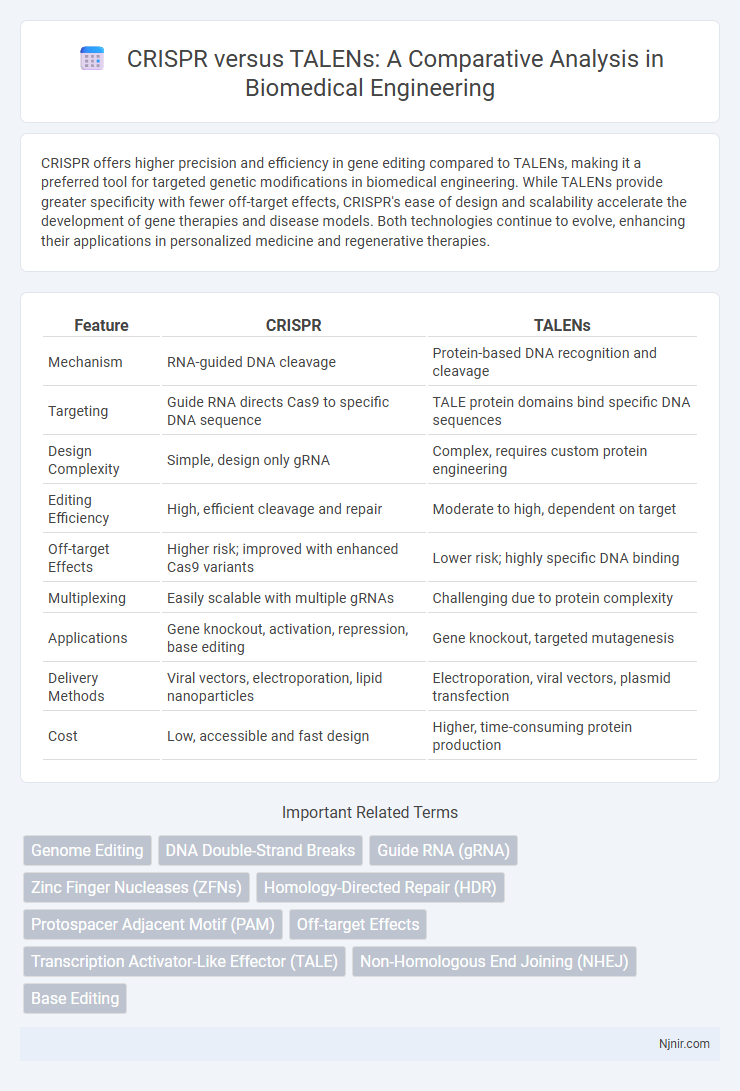
CRISPR offers higher precision and efficiency in gene editing compared to TALENs, making it a preferred tool for targeted genetic modifications in biomedical engineering. While TALENs provide greater specificity with fewer off-target effects, CRISPR's ease of design and scalability accelerate the development of gene therapies and disease models. Both technologies continue to evolve, enhancing their applications in personalized medicine and regenerative therapies.
Table of Comparison
| Feature | CRISPR | TALENs |
|---|---|---|
| Mechanism | RNA-guided DNA cleavage | Protein-based DNA recognition and cleavage |
| Targeting | Guide RNA directs Cas9 to specific DNA sequence | TALE protein domains bind specific DNA sequences |
| Design Complexity | Simple, design only gRNA | Complex, requires custom protein engineering |
| Editing Efficiency | High, efficient cleavage and repair | Moderate to high, dependent on target |
| Off-target Effects | Higher risk; improved with enhanced Cas9 variants | Lower risk; highly specific DNA binding |
| Multiplexing | Easily scalable with multiple gRNAs | Challenging due to protein complexity |
| Applications | Gene knockout, activation, repression, base editing | Gene knockout, targeted mutagenesis |
| Delivery Methods | Viral vectors, electroporation, lipid nanoparticles | Electroporation, viral vectors, plasmid transfection |
| Cost | Low, accessible and fast design | Higher, time-consuming protein production |
Introduction to Genome Editing Technologies
CRISPR and TALENs represent two pivotal genome editing technologies that enable targeted DNA modifications with high precision. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) utilizes a guide RNA to direct the Cas9 endonuclease to specific genomic loci, facilitating efficient and versatile gene editing. TALENs (Transcription Activator-Like Effector Nucleases) rely on customizable DNA-binding domains fused to a nuclease to induce double-strand breaks, offering specificity but with more complex design requirements compared to CRISPR.
Overview of CRISPR-Cas Systems
CRISPR-Cas systems are adaptive immune mechanisms in bacteria and archaea that utilize RNA-guided nucleases, such as Cas9, to identify and cleave specific DNA sequences. This technology enables precise genome editing by designing guide RNAs complementary to target DNA, offering higher efficiency and easier programmability compared to TALENs, which rely on engineered protein-DNA interactions. The simplicity of CRISPR's RNA-based targeting mechanism accelerates genetic research and therapeutic applications, highlighting its dominance in modern gene editing.
Understanding TALENs Technology
TALENs (Transcription Activator-Like Effector Nucleases) technology utilizes engineered proteins to target specific DNA sequences for precise gene editing by fusing DNA-binding domains with a nuclease domain. The modular nature of TALENs allows for customizable recognition of nucleotides through repeat-variable diresidues (RVDs), enabling high specificity and minimizing off-target effects. This platform is particularly effective for editing complex genomes and is valuable in therapeutic applications where precision is critical.
Mechanisms of DNA Targeting: CRISPR vs TALENs
CRISPR uses a guide RNA to locate specific DNA sequences, directing the Cas9 endonuclease to induce double-strand breaks precisely at the target site. TALENs rely on engineered transcription activator-like effector domains that recognize and bind specific DNA bases, coupled with FokI nuclease domains that dimerize to cleave DNA. The RNA-guided mechanism of CRISPR offers greater flexibility and efficiency compared to the protein-DNA interactions required for TALEN targeting.
Efficiency and Precision in Genome Editing
CRISPR systems offer higher efficiency in genome editing due to their simpler design and ease of programming with guide RNA, enabling rapid targeting of multiple genes simultaneously. TALENs provide exceptional precision and lower off-target effects as their DNA-binding domains recognize longer sequences, reducing unintended mutations. Both technologies excel in specific applications where CRISPR favors high-throughput edits and TALENs are preferred for precise, single-gene modifications.
Off-Target Effects and Specificity
CRISPR technology exhibits higher efficiency but often shows increased off-target effects compared to TALENs, which provide greater specificity due to their longer DNA recognition sequences. TALENs' modular design allows precise targeting of unique genomic sites, minimizing unintended modifications in gene editing applications. Advances in engineered Cas variants aim to enhance CRISPR specificity, yet TALENs remain advantageous in contexts demanding stringent off-target control.
Delivery Methods and Cellular Compatibility
CRISPR systems typically leverage viral vectors like AAV and lentivirus for efficient delivery, along with non-viral methods such as lipid nanoparticles, offering broad cellular compatibility and ease of multiplexing. TALENs rely more on plasmid DNA, mRNA, or protein delivery through electroporation or microinjection, with higher specificity but often limited delivery efficiency in certain difficult-to-transfect cell types. While CRISPR excels in adaptability across diverse cellular environments, TALENs provide precise genome editing with lower off-target effects, though cellular uptake remains more challenging in primary cells and in vivo applications.
Biomedical Applications: Therapeutic Potential
CRISPR offers a highly efficient and versatile platform for targeted gene editing, enabling the correction of genetic disorders such as sickle cell anemia and cystic fibrosis with unprecedented precision. TALENs provide comparable specificity and low off-target effects, making them valuable for therapeutic applications where minimizing unintended mutations is critical, including cancer immunotherapy and inherited retinal diseases. Both technologies advance personalized medicine by facilitating the development of gene therapies that can be tailored to individual patient genetic profiles, expanding treatment options for previously untreatable conditions.
Challenges and Limitations
CRISPR faces challenges related to off-target effects and delivery efficiency, which can limit its precision and therapeutic applications. TALENs offer high specificity but suffer from complex design and labor-intensive assembly processes, making scalability difficult. Both gene-editing technologies encounter limitations in targeting repetitive DNA sequences and inducing unintended immune responses.
Future Perspectives in Genome Engineering
CRISPR technology offers unparalleled efficiency and ease of use compared to TALENs, driving widespread adoption in genome engineering and accelerating therapeutic development. Despite TALENs' higher specificity and lower off-target effects, CRISPR's continual improvements in precision, such as base editing and prime editing, position it as the dominant tool for future gene therapies. Emerging hybrid systems integrating CRISPR and TALEN components may further enhance accuracy, expanding applications in personalized medicine and complex genome modifications.
Genome Editing
CRISPR offers faster, more precise genome editing with simpler design and higher efficiency compared to TALENs, which require complex protein engineering for targeted DNA modifications.
DNA Double-Strand Breaks
CRISPR generates targeted DNA double-strand breaks using RNA-guided Cas9 nuclease, while TALENs induce breaks via engineered protein-DNA interactions with FokI nuclease domains.
Guide RNA (gRNA)
CRISPR utilizes a single-guide RNA (gRNA) that directs the Cas9 enzyme to specific DNA sequences with high precision, whereas TALENs rely on engineered proteins for DNA recognition, making CRISPR's gRNA-based targeting more flexible and customizable.
Zinc Finger Nucleases (ZFNs)
Zinc Finger Nucleases (ZFNs) offer targeted genome editing by combining a zinc finger DNA-binding domain with a FokI nuclease, providing precision similar to TALENs but with smaller protein size and higher delivery efficiency compared to CRISPR systems.
Homology-Directed Repair (HDR)
CRISPR enables higher efficiency and precision in Homology-Directed Repair (HDR) compared to TALENs due to its simpler design and RNA-guided targeting mechanism.
Protospacer Adjacent Motif (PAM)
CRISPR relies on the presence of a specific Protospacer Adjacent Motif (PAM) sequence for target recognition and cleavage, whereas TALENs do not require a PAM, enabling more flexible DNA targeting.
Off-target Effects
CRISPR exhibits higher off-target effects compared to TALENs due to its RNA-guided DNA recognition mechanism, whereas TALENs demonstrate greater specificity by using engineered protein-DNA interactions.
Transcription Activator-Like Effector (TALE)
Transcription Activator-Like Effectors (TALEs) enable precise genome editing through customizable DNA-binding domains, offering targeted modifications with high specificity compared to CRISPR systems.
Non-Homologous End Joining (NHEJ)
CRISPR enables more efficient and precise induction of double-strand breaks for Non-Homologous End Joining (NHEJ) repair compared to TALENs, resulting in higher gene editing accuracy and reduced off-target effects.
Base Editing
CRISPR base editing enables precise single-nucleotide changes without double-strand breaks, offering higher efficiency and specificity compared to TALENs, which rely on DNA cleavage and repair.
CRISPR vs TALENs Infographic
 njnir.com
njnir.com