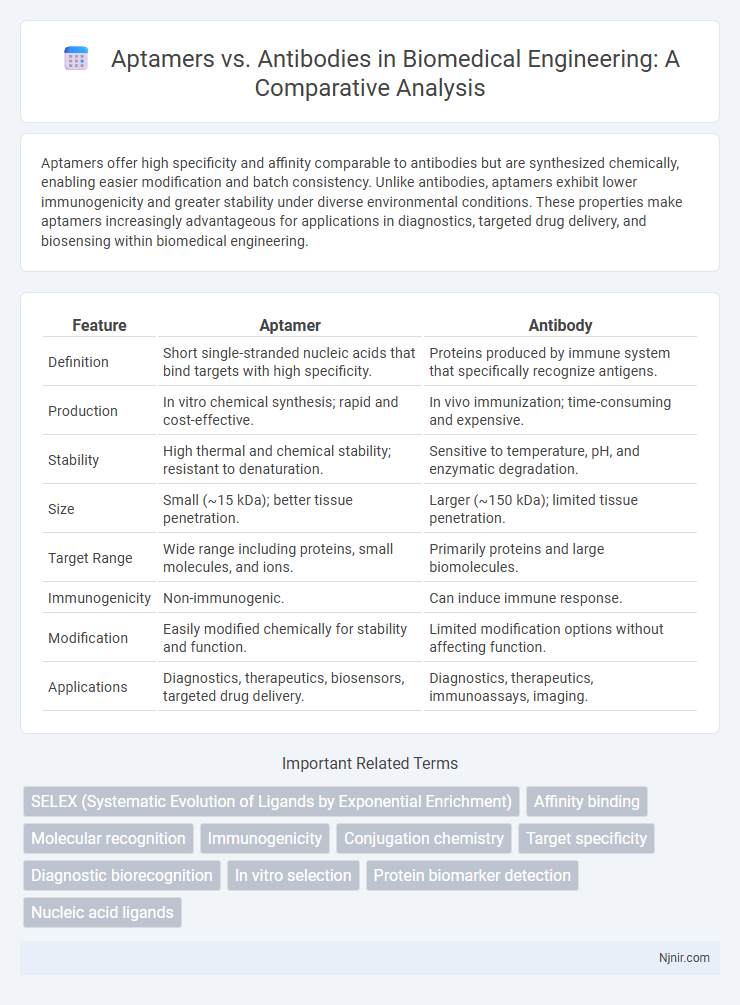
Aptamers offer high specificity and affinity comparable to antibodies but are synthesized chemically, enabling easier modification and batch consistency. Unlike antibodies, aptamers exhibit lower immunogenicity and greater stability under diverse environmental conditions. These properties make aptamers increasingly advantageous for applications in diagnostics, targeted drug delivery, and biosensing within biomedical engineering.
Table of Comparison
| Feature | Aptamer | Antibody |
|---|---|---|
| Definition | Short single-stranded nucleic acids that bind targets with high specificity. | Proteins produced by immune system that specifically recognize antigens. |
| Production | In vitro chemical synthesis; rapid and cost-effective. | In vivo immunization; time-consuming and expensive. |
| Stability | High thermal and chemical stability; resistant to denaturation. | Sensitive to temperature, pH, and enzymatic degradation. |
| Size | Small (~15 kDa); better tissue penetration. | Larger (~150 kDa); limited tissue penetration. |
| Target Range | Wide range including proteins, small molecules, and ions. | Primarily proteins and large biomolecules. |
| Immunogenicity | Non-immunogenic. | Can induce immune response. |
| Modification | Easily modified chemically for stability and function. | Limited modification options without affecting function. |
| Applications | Diagnostics, therapeutics, biosensors, targeted drug delivery. | Diagnostics, therapeutics, immunoassays, imaging. |
Introduction to Aptamers and Antibodies
Aptamers are short, single-stranded DNA or RNA molecules that fold into unique three-dimensional shapes, enabling them to bind specifically to target molecules, including proteins, small molecules, and cells. Antibodies, produced by B-cells in the immune system, are large Y-shaped proteins that recognize and bind with high affinity to antigens, playing a crucial role in immune defense and diagnostic applications. Both aptamers and antibodies serve as molecular recognition elements in biosensing, therapeutics, and diagnostics, but differ in structure, stability, and production methods.
Structural Differences: Aptamers vs Antibodies
Aptamers are short, single-stranded nucleic acids that fold into unique three-dimensional shapes, enabling specific binding to target molecules, while antibodies are large, Y-shaped proteins composed of two heavy and two light polypeptide chains forming variable regions for antigen recognition. Aptamers exhibit lower molecular weight (typically 5-15 kDa) compared to antibodies (~150 kDa), providing advantages in tissue penetration and synthetic versatility. The structural flexibility of aptamers allows rapid conformational changes upon target interaction, contrasting with the relatively rigid and stable framework of antibodies maintained by disulfide bonds.
Binding Affinity and Specificity Comparison
Aptamers exhibit high binding affinity comparable to antibodies, often in the low nanomolar to picomolar range, enabling precise target recognition. Their synthetic nature allows for greater specificity with reduced cross-reactivity, minimizing off-target interactions common in antibodies. Unlike antibodies, aptamers maintain consistent binding performance under a broader range of conditions due to their chemical stability and modifiability.
Stability and Storage Considerations
Aptamers exhibit superior stability compared to antibodies, maintaining functionality under a wider range of temperatures and pH conditions, which simplifies storage requirements. Unlike antibodies that often require refrigeration and are prone to denaturation and aggregation, aptamers can be stored at room temperature for extended periods without significant loss of activity. This enhanced stability reduces logistics costs and improves shelf-life, making aptamers highly advantageous for diagnostic and therapeutic applications.
Synthesis and Production Processes
Aptamers are synthesized through automated chemical processes using SELEX (Systematic Evolution of Ligands by Exponential Enrichment), allowing rapid, cost-effective production with high purity and batch-to-batch consistency. Antibodies require biological systems such as hybridoma technology or recombinant DNA methods, making production time-consuming, costly, and subject to variability due to reliance on animal hosts or cell culture. The synthetic nature of aptamers enables precise modifications and scalability, contrasting with the complex and variable protein folding and post-translational modifications inherent in antibody production.
Cost-Effectiveness in Biomedical Applications
Aptamers offer a cost-effective alternative to antibodies in biomedical applications due to their lower production costs and chemical synthesis scalability, reducing batch-to-batch variability and enabling rapid, high-throughput manufacturing. Their stability at room temperature and longer shelf life further decrease storage and transportation expenses compared to antibodies, which often require cold chain logistics. These attributes make aptamers economically advantageous in diagnostic assays, targeted drug delivery, and biosensor development, optimizing overall biomedical research and clinical budgets.
Diagnostic Applications: Aptamers vs Antibodies
Aptamers offer high specificity and stability in diagnostic applications, enabling rapid and sensitive detection of biomarkers compared to antibodies. Unlike antibodies, aptamers are synthesized chemically, providing batch-to-batch consistency and reduced production costs. Their small size ensures better tissue penetration and minimal immunogenicity, making them ideal for point-of-care diagnostic devices.
Therapeutic Potential: Benefits and Limitations
Aptamers offer high specificity and low immunogenicity in therapeutic applications, enabling targeted delivery with reduced side effects compared to antibodies. Antibodies provide robust stability and well-established clinical use but may cause immune reactions and require complex production methods. Despite aptamers' rapid synthesis and ease of modification, their susceptibility to nuclease degradation limits in vivo stability compared to antibodies.
Challenges in Clinical Translation
Aptamers face challenges in clinical translation due to their susceptibility to nuclease degradation and rapid renal clearance, requiring chemical modifications to enhance stability and bioavailability. Antibodies, while more stable, encounter issues with immunogenicity, batch-to-batch variability, and high production costs, limiting their widespread clinical use. Both modalities require optimized delivery systems and rigorous validation to overcome these hurdles for effective therapeutic and diagnostic applications.
Future Perspectives in Biomedical Engineering
Aptamers offer significant future potential in biomedical engineering due to their high specificity, low immunogenicity, and ease of synthesis compared to antibodies. Advances in SELEX technology and nanotechnology integration are driving the development of aptamer-based biosensors, targeted drug delivery systems, and diagnostics with enhanced sensitivity and stability. Emerging applications in precision medicine and real-time biomarker monitoring highlight aptamers as promising alternatives to traditional antibodies in next-generation therapeutic and diagnostic platforms.
SELEX (Systematic Evolution of Ligands by Exponential Enrichment)
SELEX (Systematic Evolution of Ligands by Exponential Enrichment) enables aptamers to be rapidly selected with high specificity and affinity, offering a versatile and cost-effective alternative to traditional antibodies in molecular recognition applications.
Affinity binding
Aptamers exhibit high affinity binding comparable to antibodies, with dissociation constants typically in the low nanomolar to picomolar range, enabling precise and selective target recognition.
Molecular recognition
Aptamers demonstrate high molecular recognition specificity by folding into unique three-dimensional structures that bind target molecules with affinity comparable to antibodies but offer advantages such as chemical synthesis and stability.
Immunogenicity
Aptamers exhibit significantly lower immunogenicity compared to antibodies, making them safer and more suitable for repeated therapeutic use in immune-sensitive applications.
Conjugation chemistry
Aptamer conjugation chemistry offers higher specificity and stability through site-specific modifications using click chemistry and thiol-maleimide reactions, surpassing traditional antibody conjugation methods limited by random lysine or cysteine coupling.
Target specificity
Aptamers exhibit higher target specificity and affinity compared to antibodies due to their unique nucleic acid structures enabling precise molecular recognition.
Diagnostic biorecognition
Aptamers offer higher stability, lower production costs, and improved reusability compared to antibodies, making them increasingly effective biorecognition elements in diagnostic applications.
In vitro selection
Aptamers are synthesized through in vitro selection processes such as SELEX, enabling rapid identification of high-affinity ligands, whereas antibodies require in vivo immunization and hybridoma technology for production.
Protein biomarker detection
Aptamers offer higher specificity and stability than antibodies for protein biomarker detection, enabling more sensitive and rapid diagnostic assays.
Nucleic acid ligands
Nucleic acid ligands such as aptamers offer higher specificity, easier synthesis, and improved stability compared to traditional protein antibodies.
Aptamer vs Antibody Infographic
 njnir.com
njnir.com