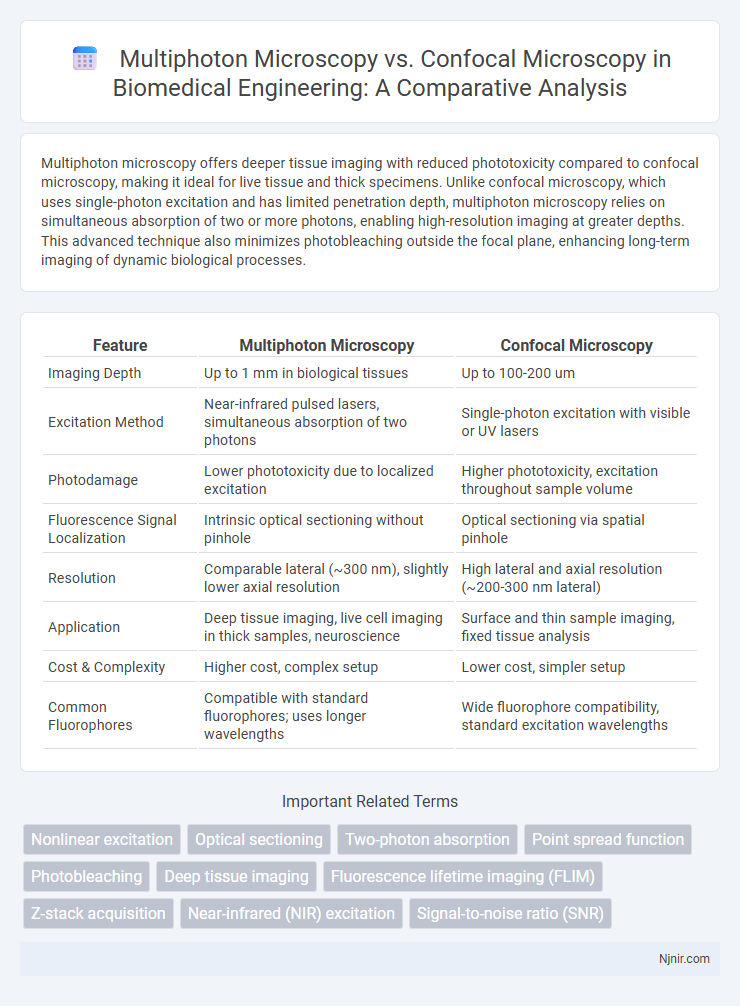
Multiphoton microscopy offers deeper tissue imaging with reduced phototoxicity compared to confocal microscopy, making it ideal for live tissue and thick specimens. Unlike confocal microscopy, which uses single-photon excitation and has limited penetration depth, multiphoton microscopy relies on simultaneous absorption of two or more photons, enabling high-resolution imaging at greater depths. This advanced technique also minimizes photobleaching outside the focal plane, enhancing long-term imaging of dynamic biological processes.
Table of Comparison
| Feature | Multiphoton Microscopy | Confocal Microscopy |
|---|---|---|
| Imaging Depth | Up to 1 mm in biological tissues | Up to 100-200 um |
| Excitation Method | Near-infrared pulsed lasers, simultaneous absorption of two photons | Single-photon excitation with visible or UV lasers |
| Photodamage | Lower phototoxicity due to localized excitation | Higher phototoxicity, excitation throughout sample volume |
| Fluorescence Signal Localization | Intrinsic optical sectioning without pinhole | Optical sectioning via spatial pinhole |
| Resolution | Comparable lateral (~300 nm), slightly lower axial resolution | High lateral and axial resolution (~200-300 nm lateral) |
| Application | Deep tissue imaging, live cell imaging in thick samples, neuroscience | Surface and thin sample imaging, fixed tissue analysis |
| Cost & Complexity | Higher cost, complex setup | Lower cost, simpler setup |
| Common Fluorophores | Compatible with standard fluorophores; uses longer wavelengths | Wide fluorophore compatibility, standard excitation wavelengths |
Introduction to Biomedical Imaging Modalities
Multiphoton microscopy offers deeper tissue penetration and reduced phototoxicity compared to confocal microscopy, making it ideal for live-cell and in vivo imaging applications. Confocal microscopy provides high-resolution optical sectioning by using point illumination and pinhole detection to eliminate out-of-focus light, suitable for thin tissue samples and fixed cells. Both imaging modalities leverage laser scanning techniques but differ in excitation methods, with multiphoton relying on longer wavelength pulsed lasers and confocal using single-photon excitation.
Principles of Confocal Microscopy
Confocal microscopy employs point illumination and a spatial pinhole to eliminate out-of-focus light, enhancing optical resolution and contrast in thick specimens. It utilizes laser light scanning and fluorescence detection to produce sharp, high-resolution images from specific focal planes within a sample. This principle allows for three-dimensional reconstruction but is limited by photobleaching and penetration depth compared to multiphoton microscopy.
Mechanism of Multiphoton Microscopy
Multiphoton microscopy employs near-infrared laser pulses to excite fluorophores through simultaneous absorption of two or more photons, enabling deeper tissue penetration and reduced photodamage compared to confocal microscopy. This nonlinear excitation mechanism confines fluorescence to the focal volume, enhancing image contrast and spatial resolution in thick biological samples. Unlike confocal microscopy, which uses single-photon excitation with visible light and pinhole detection to reject out-of-focus light, multiphoton microscopy allows imaging at depths exceeding 500 micrometers in scattering tissues.
Resolution and Imaging Depth Comparison
Multiphoton microscopy achieves superior imaging depth up to 1 millimeter in biological tissues due to longer excitation wavelengths and reduced scattering, whereas confocal microscopy typically penetrates up to 100-200 micrometers. In terms of resolution, confocal microscopy offers lateral resolution around 200 nanometers and axial resolution near 500 nanometers, while multiphoton microscopy presents slightly lower lateral resolution but comparable axial resolution due to its nonlinear excitation process. Multiphoton microscopy's advantage in deeper tissue imaging makes it ideal for in vivo studies, whereas confocal microscopy excels in high-resolution surface imaging.
Fluorescence Excitation and Phototoxicity
Multiphoton microscopy utilizes longer-wavelength, near-infrared light for fluorescence excitation, which reduces phototoxicity and enables deeper tissue imaging compared to confocal microscopy that employs shorter-wavelength, visible light leading to higher photodamage. The localized excitation in multiphoton microscopy confines fluorescence to the focal point, minimizing out-of-focus excitation and photobleaching, whereas confocal microscopy relies on pinhole apertures to reject out-of-focus light but can still cause significant phototoxicity due to excitation throughout the beam path. Consequently, multiphoton microscopy is preferred for live-cell imaging and thick biological specimens where reduced photodamage and increased penetration depth are critical.
Live Tissue and In Vivo Imaging Capabilities
Multiphoton microscopy enables deeper imaging of live tissue with reduced phototoxicity and photobleaching compared to confocal microscopy, making it ideal for in vivo studies of thick specimens such as brain or tumor tissues. Confocal microscopy provides high-resolution images with excellent optical sectioning but is limited by shallower penetration depth and increased photodamage in live tissue imaging. Multiphoton's use of near-infrared excitation wavelengths enhances tissue penetration and allows long-term imaging of physiological processes in living organisms.
Signal-to-Noise Ratio and Image Quality
Multiphoton microscopy offers superior signal-to-noise ratio compared to confocal microscopy due to its longer excitation wavelengths and confined excitation volume, which minimize out-of-focus fluorescence and photodamage. This technique enhances image quality by enabling deeper tissue penetration and producing high-contrast, high-resolution images in thick biological samples. Confocal microscopy, while effective for surface-level imaging, often suffers from reduced signal-to-noise ratio and image clarity at greater depths due to scattering and autofluorescence.
Equipment Cost and Accessibility
Multiphoton microscopy generally requires more expensive laser systems, such as femtosecond pulsed lasers, making the initial equipment cost significantly higher than confocal microscopy, which uses less costly continuous-wave lasers. The complexity and maintenance of multiphoton setups also limit accessibility to well-funded research institutions, whereas confocal microscopy is more widely available due to lower costs and simpler operation. Laboratories prioritizing budget constraints often choose confocal microscopy for routine imaging, while multiphoton microscopy is selected for deep tissue imaging despite its financial and logistical demands.
Applications in Biomedical Research
Multiphoton microscopy enables deep tissue imaging with reduced phototoxicity, making it ideal for live animal studies and neuroscience applications where prolonged observation is crucial. Confocal microscopy provides high-resolution imaging of thin, fixed tissue sections, widely used in cellular and molecular biology for detailed structural analysis. Both techniques complement each other, with multiphoton preferred for in vivo imaging and confocal excelling in high-contrast visualization of fluorescently labeled specimens.
Future Trends in Optical Microscopy
Multiphoton microscopy offers deeper tissue penetration and reduced phototoxicity compared to confocal microscopy, making it ideal for live tissue imaging and neuroscience research. Advances in laser technology and adaptive optics are enhancing resolution and imaging speed for both modalities, driving new applications in real-time, high-resolution in vivo studies. Integration with artificial intelligence and multimodal imaging platforms is expected to revolutionize data acquisition and analysis, enabling more precise and comprehensive biological insights.
Nonlinear excitation
Multiphoton microscopy uses nonlinear excitation to achieve deeper tissue imaging with reduced photodamage compared to confocal microscopy's linear excitation approach.
Optical sectioning
Multiphoton microscopy enables deeper optical sectioning with reduced photodamage and scattering compared to confocal microscopy, making it ideal for high-resolution imaging in thick biological tissues.
Two-photon absorption
Multiphoton microscopy utilizes two-photon absorption to enable deeper tissue imaging with reduced photodamage compared to confocal microscopy's single-photon excitation.
Point spread function
Multiphoton microscopy offers a superior point spread function with enhanced axial resolution and reduced photobleaching compared to confocal microscopy, enabling deeper tissue imaging with higher signal-to-noise ratio.
Photobleaching
Multiphoton microscopy significantly reduces photobleaching compared to confocal microscopy by confining excitation to the focal plane using longer wavelengths, preserving sample integrity during deep tissue imaging.
Deep tissue imaging
Multiphoton microscopy enables deeper tissue imaging up to several hundred micrometers with reduced phototoxicity compared to confocal microscopy, which is typically limited to imaging depths of 100-200 micrometers due to higher scattering and photodamage.
Fluorescence lifetime imaging (FLIM)
Multiphoton microscopy offers deeper tissue penetration and reduced photobleaching for Fluorescence Lifetime Imaging Microscopy (FLIM) compared to confocal microscopy, enabling more accurate lifetime measurements in thick biological samples.
Z-stack acquisition
Multiphoton microscopy enables deeper Z-stack acquisition with reduced photodamage and improved imaging of thick tissues compared to confocal microscopy's limited penetration and higher photobleaching.
Near-infrared (NIR) excitation
Multiphoton microscopy uses near-infrared (NIR) excitation to achieve deeper tissue penetration and reduced photodamage compared to confocal microscopy, which relies on visible light excitation and has limited imaging depth.
Signal-to-noise ratio (SNR)
Multiphoton microscopy offers a higher signal-to-noise ratio (SNR) than confocal microscopy due to its reduced out-of-focus fluorescence and deeper tissue penetration.
Multiphoton microscopy vs Confocal microscopy Infographic
 njnir.com
njnir.com