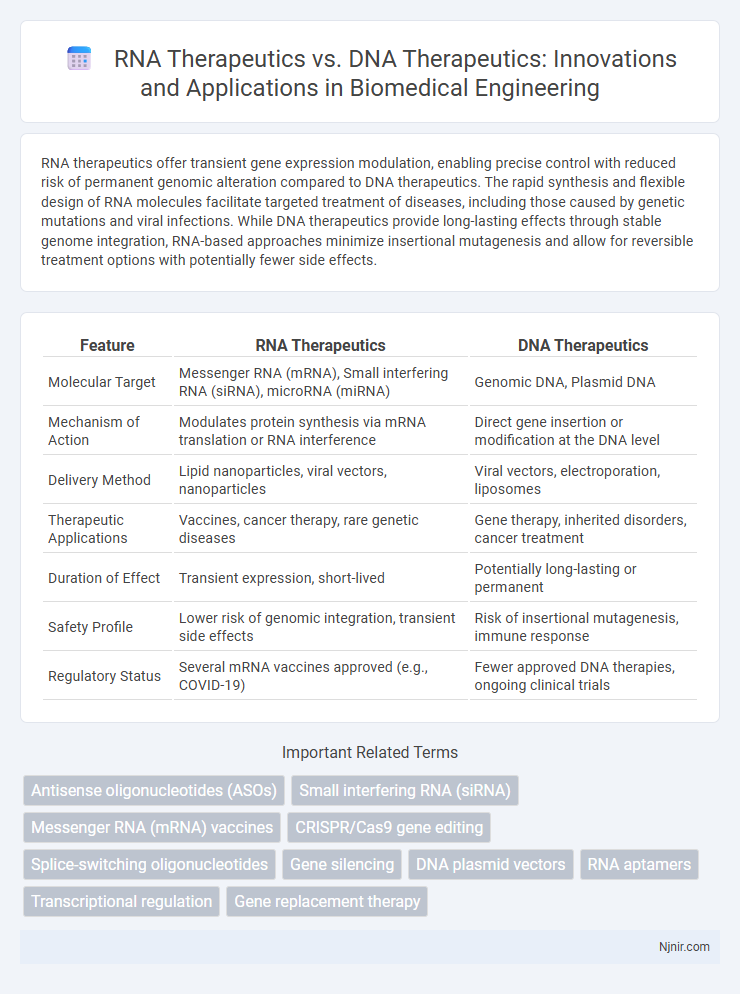
RNA therapeutics offer transient gene expression modulation, enabling precise control with reduced risk of permanent genomic alteration compared to DNA therapeutics. The rapid synthesis and flexible design of RNA molecules facilitate targeted treatment of diseases, including those caused by genetic mutations and viral infections. While DNA therapeutics provide long-lasting effects through stable genome integration, RNA-based approaches minimize insertional mutagenesis and allow for reversible treatment options with potentially fewer side effects.
Table of Comparison
| Feature | RNA Therapeutics | DNA Therapeutics |
|---|---|---|
| Molecular Target | Messenger RNA (mRNA), Small interfering RNA (siRNA), microRNA (miRNA) | Genomic DNA, Plasmid DNA |
| Mechanism of Action | Modulates protein synthesis via mRNA translation or RNA interference | Direct gene insertion or modification at the DNA level |
| Delivery Method | Lipid nanoparticles, viral vectors, nanoparticles | Viral vectors, electroporation, liposomes |
| Therapeutic Applications | Vaccines, cancer therapy, rare genetic diseases | Gene therapy, inherited disorders, cancer treatment |
| Duration of Effect | Transient expression, short-lived | Potentially long-lasting or permanent |
| Safety Profile | Lower risk of genomic integration, transient side effects | Risk of insertional mutagenesis, immune response |
| Regulatory Status | Several mRNA vaccines approved (e.g., COVID-19) | Fewer approved DNA therapies, ongoing clinical trials |
Introduction to Nucleic Acid-Based Therapeutics
Nucleic acid-based therapeutics encompass RNA and DNA therapies that target genetic information to treat diseases at the molecular level. RNA therapeutics, including mRNA vaccines and siRNA, facilitate transient gene expression modulation or silencing, offering rapid and reversible treatment options. DNA therapeutics involve gene editing or replacement strategies such as CRISPR or plasmid DNA, enabling long-term genetic corrections but often requiring more complex delivery systems.
Mechanisms of Action: RNA vs DNA Therapeutics
RNA therapeutics primarily function by modulating gene expression through mechanisms such as RNA interference (RNAi), antisense oligonucleotides, and mRNA-based protein replacement, enabling transient and reversible effects. DNA therapeutics involve direct modification or addition of genetic material via gene editing techniques like CRISPR-Cas9 or viral and non-viral gene delivery systems, resulting in potentially permanent genomic changes. RNA-based therapies offer rapid response and reduced risk of insertional mutagenesis, while DNA therapies provide long-lasting treatment outcomes by altering the genome itself.
Key Delivery Strategies for RNA and DNA Agents
RNA therapeutics primarily utilize lipid nanoparticles (LNPs) and electroporation to efficiently deliver RNA molecules into target cells, enhancing stability and cellular uptake. DNA therapeutics often rely on viral vectors like adeno-associated viruses (AAV) and non-viral methods such as plasmid DNA delivery through electroporation or gene gun techniques to achieve efficient gene expression. Both strategies focus on overcoming biological barriers, optimizing carrier design, and ensuring targeted release to maximize therapeutic efficacy.
Therapeutic Targets: Genomic vs Transcriptomic Interventions
RNA therapeutics primarily target transcriptomic components by modulating mRNA to regulate protein expression, enabling reversible and dynamic intervention in gene expression pathways. DNA therapeutics focus on genomic targets by directly editing or inserting genetic sequences within the genome, offering permanent or long-lasting genetic corrections. This distinction governs clinical strategies, where RNA therapies address transient biological processes, while DNA therapies aim for durable genomic modifications.
Clinical Applications of RNA-based Therapeutics
RNA-based therapeutics have demonstrated significant potential in clinical applications such as mRNA vaccines for infectious diseases, RNA interference (RNAi) for gene silencing in genetic disorders, and antisense oligonucleotides for modulating RNA splicing in rare diseases. These therapies offer advantages like rapid development, transient expression, and reduced risk of genomic integration compared to DNA-based therapeutics. Current clinical trials explore RNA therapeutics in oncology, rare genetic diseases, and viral infections, highlighting their expanding role in precision medicine.
Clinical Applications of DNA-based Therapeutics
DNA-based therapeutics harness gene editing tools such as CRISPR and viral vector delivery systems to treat genetic disorders, cancers, and infectious diseases by directly modifying or supplementing the genome. Clinical applications include gene replacement therapies for inherited conditions like cystic fibrosis and muscular dystrophy, as well as DNA vaccines that stimulate adaptive immunity against pathogens. Ongoing clinical trials demonstrate the potential of DNA therapeutics to provide long-lasting effects and personalized medicine solutions through precise genomic interventions.
Safety and Immunogenicity: Comparing RNA and DNA Therapies
RNA therapeutics present a lower risk of genomic integration compared to DNA therapies, enhancing their safety profile by minimizing potential insertional mutagenesis. DNA therapeutics often require nuclear entry, increasing the risk of immune recognition and subsequent inflammatory responses, while RNA therapeutics act predominantly in the cytoplasm with reduced immunogenicity. Advances in nucleoside modifications and delivery systems further decrease immune activation in RNA therapies, making them preferable for applications demanding high safety standards.
Manufacturing and Scalability Challenges
RNA therapeutics face manufacturing challenges due to their inherent instability, requiring stringent cold-chain logistics and complex lipid nanoparticle formulations for effective delivery. DNA therapeutics present scalability issues linked to the need for larger-scale vector production, such as viral vectors or plasmids, which demand costly and time-consuming bioprocessing techniques. Both modalities require specialized facilities and quality control measures, but RNA platforms generally offer faster batch production times compared to DNA-based manufacturing.
Regulatory and Ethical Considerations
RNA therapeutics face distinct regulatory challenges due to their transient nature and potential for off-target effects, requiring rigorous safety evaluations by agencies like the FDA and EMA. DNA therapeutics, involving permanent genetic modifications, raise heightened ethical concerns related to germline editing and long-term impacts, demanding stricter oversight and informed consent protocols. Regulatory frameworks prioritize patient safety, efficacy, and ethical adherence, balancing innovation with potential risks inherent in genomic interventions.
Future Perspectives in Nucleic Acid Therapeutics
RNA therapeutics exhibit rapid development due to their ability to transiently modulate gene expression with high specificity, minimizing long-term genomic alterations compared to DNA therapeutics. Advances in delivery platforms and chemical modifications are enhancing RNA stability and cellular uptake, positioning RNA-based treatments as frontrunners for addressing genetic disorders, cancers, and viral infections. Future perspectives emphasize expanding personalized medicine applications, integrating CRISPR-based RNA editing, and overcoming immune response challenges to fully realize the therapeutic potential of nucleic acids.
Antisense oligonucleotides (ASOs)
Antisense oligonucleotides (ASOs) in RNA therapeutics precisely target and modulate RNA sequences to regulate gene expression, offering reversible and direct intervention compared to DNA therapeutics that involve permanent genomic alterations.
Small interfering RNA (siRNA)
Small interfering RNA (siRNA) therapeutics offer precise post-transcriptional gene silencing by targeting mRNA degradation, unlike DNA therapeutics which focus on genomic modification or gene replacement.
Messenger RNA (mRNA) vaccines
Messenger RNA (mRNA) vaccines revolutionize RNA therapeutics by enabling rapid, transient protein expression without altering DNA, offering safer, faster, and more adaptable treatments compared to DNA therapeutics.
CRISPR/Cas9 gene editing
CRISPR/Cas9 gene editing enables precise, efficient RNA-guided DNA modifications for DNA therapeutics, while RNA therapeutics leverage transient, controllable RNA molecules for protein expression without permanent genome changes.
Splice-switching oligonucleotides
Splice-switching oligonucleotides in RNA therapeutics offer precise modulation of pre-mRNA splicing to correct genetic defects, whereas DNA therapeutics typically target genome editing or gene replacement with broader genomic alterations.
Gene silencing
RNA therapeutics utilize mechanisms like RNA interference to achieve gene silencing by degrading target mRNA, whereas DNA therapeutics primarily involve gene replacement or editing without directly causing gene silencing.
DNA plasmid vectors
DNA plasmid vectors in therapeutics offer stable gene expression and lower immunogenicity compared to RNA therapeutics, enabling long-term genetic treatments and efficient delivery in gene therapy applications.
RNA aptamers
RNA aptamers in RNA therapeutics offer high specificity and rapid development compared to DNA therapeutics, enabling targeted treatment with reduced immunogenicity and enhanced cellular uptake.
Transcriptional regulation
RNA therapeutics modulate gene expression post-transcriptionally by targeting mRNA stability and translation, while DNA therapeutics influence transcriptional regulation directly through genome editing and gene insertion to alter gene expression patterns.
Gene replacement therapy
RNA therapeutics offer transient gene expression with reduced risk of genomic integration, while DNA therapeutics enable long-term gene replacement but carry higher risks of insertional mutagenesis.
RNA therapeutics vs DNA therapeutics Infographic
 njnir.com
njnir.com