Microfluidics enables precise manipulation of tiny fluid volumes, facilitating high-throughput biological experiments with minimal reagent use. Organs-on-chips integrate microfluidic channels with living cells to replicate the physiological functions of human organs, providing advanced platforms for drug testing and disease modeling. This synergy enhances the accuracy of in vitro models, bridging the gap between traditional cell cultures and complex in vivo systems.
Table of Comparison
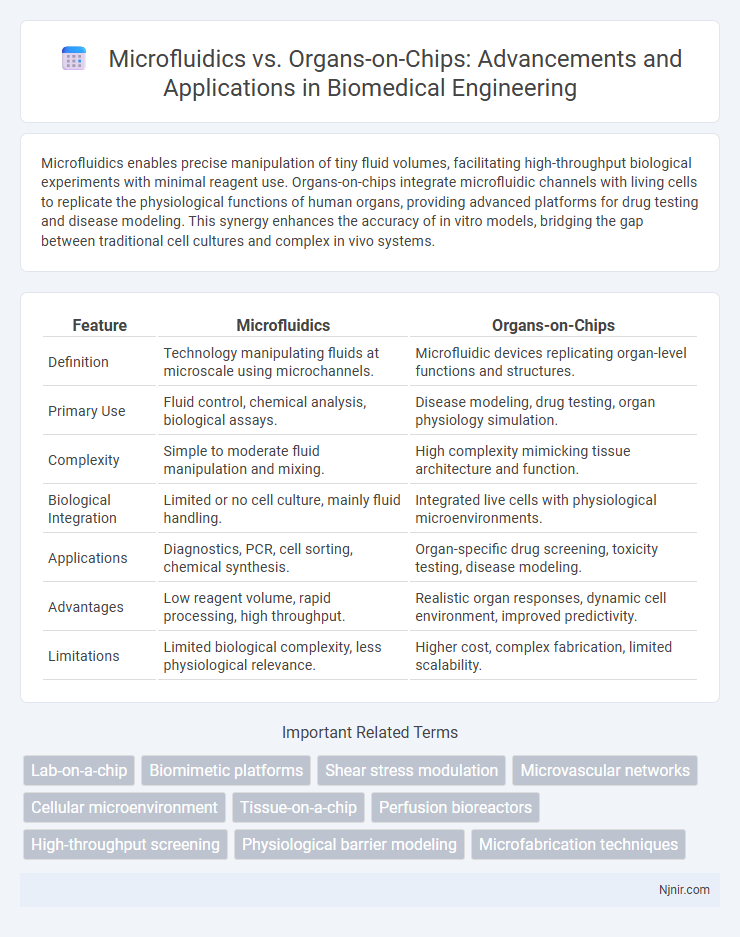
| Feature | Microfluidics | Organs-on-Chips |
|---|---|---|
| Definition | Technology manipulating fluids at microscale using microchannels. | Microfluidic devices replicating organ-level functions and structures. |
| Primary Use | Fluid control, chemical analysis, biological assays. | Disease modeling, drug testing, organ physiology simulation. |
| Complexity | Simple to moderate fluid manipulation and mixing. | High complexity mimicking tissue architecture and function. |
| Biological Integration | Limited or no cell culture, mainly fluid handling. | Integrated live cells with physiological microenvironments. |
| Applications | Diagnostics, PCR, cell sorting, chemical synthesis. | Organ-specific drug screening, toxicity testing, disease modeling. |
| Advantages | Low reagent volume, rapid processing, high throughput. | Realistic organ responses, dynamic cell environment, improved predictivity. |
| Limitations | Limited biological complexity, less physiological relevance. | Higher cost, complex fabrication, limited scalability. |
Introduction to Microfluidics in Biomedical Engineering
Microfluidics in biomedical engineering enables precise manipulation of fluids at the microscale, enhancing cell culture, drug delivery, and diagnostic applications. Organs-on-chips leverage microfluidic technology to replicate organ-level functions, providing dynamic, physiologically relevant models for disease study and drug testing. This integration improves human tissue simulation, surpassing traditional static cell cultures in mimicking complex biological environments.
Overview of Organs-on-Chips Technology
Organs-on-chips technology integrates microfluidics with living cells to simulate the dynamic microenvironment of human organs, enabling precise modeling of tissue functions and disease states. This platform incorporates microchannels lined with organ-specific cells, allowing controlled fluid flow and mechanical forces that mimic physiological conditions. Advanced organ-on-chip systems facilitate drug testing, disease modeling, and personalized medicine by providing high-fidelity data on organ-level responses unattainable with traditional cell cultures or animal models.
Key Principles of Microfluidics
Microfluidics involves the precise manipulation of fluids at the microliter to picoliter scale using channels typically less than 100 micrometers in diameter, enabling high surface-to-volume ratios and laminar flow conditions. Key principles include the control of fluid dynamics governed by low Reynolds numbers, capillary action, and diffusion-dominated transport, which facilitate rapid mixing and precise spatial-temporal control of biological and chemical reactions. Organs-on-chips leverage these microfluidic principles to simulate physiological environments by integrating living cells within microengineered chamber arrays to replicate organ-level functions and tissue interfaces.
Functional Design of Organs-on-Chips
The functional design of organs-on-chips integrates microfluidic technology to replicate the microenvironment of human tissues, enabling precise control over fluid flow, mechanical forces, and cellular interactions. Unlike traditional microfluidic devices that primarily manipulate fluids for chemical or biological analyses, organs-on-chips incorporate living cells in a 3D architecture to mimic organ-specific functions such as barrier permeability, tissue-tissue interfaces, and dynamic physiological responses. This sophisticated design enhances drug testing, disease modeling, and personalized medicine by providing physiologically relevant platforms that capture the complexity of human organ systems.
Comparative Analysis: Microfluidics vs Organs-on-Chips
Microfluidics technology enables precise manipulation of fluids at the microscale, facilitating high-throughput screening and controlled cellular environments. Organs-on-chips integrate microfluidic devices with living cells to replicate tissue- and organ-level functions, offering enhanced physiological relevance for drug testing and disease modeling. While microfluidics provides versatile platform engineering, organs-on-chips combine microfluidic precision with biological complexity for more accurate human organ simulation.
Applications in Drug Discovery and Toxicology Testing
Microfluidics enables precise manipulation of fluids at the microscale, facilitating high-throughput drug screening and toxicity assays with reduced reagent consumption. Organs-on-chips replicate human tissue architecture and function, providing physiologically relevant models to evaluate drug efficacy, metabolism, and safety with improved predictive accuracy compared to traditional cell culture and animal testing. Integration of microfluidics within organs-on-chips enhances dynamic control of microenvironments, accelerating drug development and reducing the reliance on costly and time-consuming clinical trials.
Challenges in Integration and Scalability
Microfluidics faces challenges in integration due to complex fluidic control and maintaining biological relevance at micro-scales, complicating the seamless incorporation of multiple functionalities. Organs-on-chips struggle with scalability issues, as replicating organ-level complexity and inter-organ interactions requires sophisticated design and extensive validation. Both technologies demand advancements in material compatibility, standardization, and manufacturing processes to enable high-throughput applications and commercial viability.
Advances in Biomimicry and Physiological Relevance
Microfluidics enables precise control of fluid dynamics at the microscale, facilitating the replication of complex biological environments within Organs-on-Chips platforms. Advances in biomimicry have led to the integration of living cells and extracellular matrices, enhancing physiological relevance by mimicking tissue-specific architecture and function. These innovations improve disease modeling and drug testing by replicating dynamic cellular interactions and microenvironmental cues with high fidelity.
Future Perspectives in Translational Medicine
Microfluidics enables precise manipulation of fluids at microscale, enhancing drug screening and disease modeling, while organs-on-chips integrate microfluidic technology with living cells to mimic complex tissue functions, providing more physiologically relevant data. The convergence of these technologies promises transformative advances in personalized medicine, enabling faster and more accurate prediction of human responses to therapies. Future developments in scalable manufacturing and integration with artificial intelligence will accelerate their adoption in translational medicine, reducing reliance on animal models and improving clinical trial success rates.
Conclusion and Outlook for Biomedical Research
Microfluidics and organs-on-chips represent transformative platforms in biomedical research by enabling precise control of cellular microenvironments and replicating organ-level functions in vitro. Microfluidics offers high-throughput screening capabilities and intricate fluid manipulation, while organs-on-chips integrate tissue engineering and microfluidic technology to simulate physiological responses more accurately. Future advancements will likely enhance personalized medicine applications, drug development efficiency, and disease modeling fidelity, driving innovations in diagnostics and therapeutic strategies.
Lab-on-a-chip
Lab-on-a-chip technology integrates microfluidics to simulate organ functions on a chip, enabling precise control of fluids for biological and chemical analyses in organ-on-chip systems.
Biomimetic platforms
Biomimetic platforms in microfluidics provide precise control of fluid dynamics and cellular microenvironments, while organs-on-chips integrate multiple tissue types to replicate complex physiological functions for advanced disease modeling and drug testing.
Shear stress modulation
Microfluidics enables precise shear stress modulation to replicate physiological flow conditions in organs-on-chips, enhancing cellular behavior and tissue function modeling.
Microvascular networks
Microfluidics enables precise control of fluid flow and microenvironment parameters essential for replicating complex microvascular networks in organs-on-chips, facilitating advanced vascular disease modeling and drug testing.
Cellular microenvironment
Microfluidics enables precise control of the cellular microenvironment by manipulating fluid flow at the microscale, while organs-on-chips integrate microfluidic technology with living cells to replicate tissue-specific microenvironments for advanced physiological modeling.
Tissue-on-a-chip
Tissue-on-a-chip technology integrates microfluidics with organ-on-chip systems to mimic complex tissue environments for improved disease modeling and drug testing.
Perfusion bioreactors
Perfusion bioreactors enhance microfluidics and organs-on-chips by providing controlled fluid flow to mimic in vivo conditions, improving cell culture viability and functional tissue modeling.
High-throughput screening
Microfluidics enables high-throughput screening by precisely manipulating fluids at the microscale, while organs-on-chips integrate microfluidic technology with living tissue to provide more physiologically relevant models for drug testing and disease research.
Physiological barrier modeling
Microfluidics enables precise control of fluid dynamics for replicating physiological barriers, while organs-on-chips integrate microfluidic systems with living cells to model complex tissue interfaces and barrier functions more accurately.
Microfabrication techniques
Microfluidics leverages advanced microfabrication techniques such as photolithography, soft lithography, and 3D printing to create precise, scalable devices, whereas organs-on-chips employ these methods to mimic complex tissue interfaces and physiological microenvironments for enhanced biomedical modeling.
Microfluidics vs Organs-on-chips Infographic
 njnir.com
njnir.com