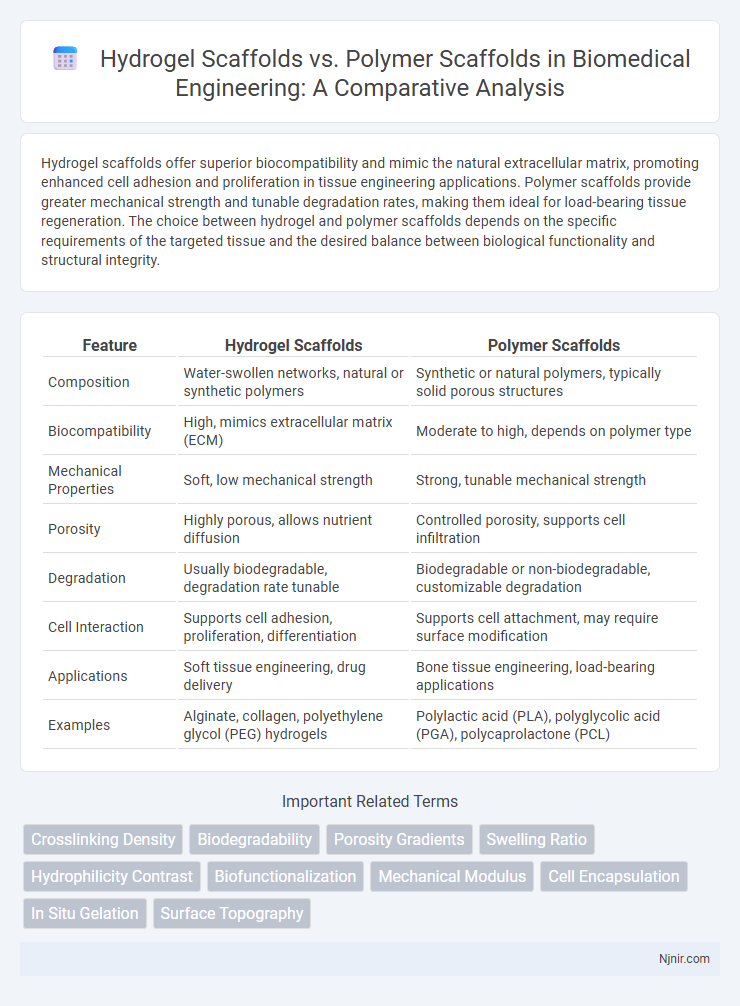
Hydrogel scaffolds offer superior biocompatibility and mimic the natural extracellular matrix, promoting enhanced cell adhesion and proliferation in tissue engineering applications. Polymer scaffolds provide greater mechanical strength and tunable degradation rates, making them ideal for load-bearing tissue regeneration. The choice between hydrogel and polymer scaffolds depends on the specific requirements of the targeted tissue and the desired balance between biological functionality and structural integrity.
Table of Comparison
| Feature | Hydrogel Scaffolds | Polymer Scaffolds |
|---|---|---|
| Composition | Water-swollen networks, natural or synthetic polymers | Synthetic or natural polymers, typically solid porous structures |
| Biocompatibility | High, mimics extracellular matrix (ECM) | Moderate to high, depends on polymer type |
| Mechanical Properties | Soft, low mechanical strength | Strong, tunable mechanical strength |
| Porosity | Highly porous, allows nutrient diffusion | Controlled porosity, supports cell infiltration |
| Degradation | Usually biodegradable, degradation rate tunable | Biodegradable or non-biodegradable, customizable degradation |
| Cell Interaction | Supports cell adhesion, proliferation, differentiation | Supports cell attachment, may require surface modification |
| Applications | Soft tissue engineering, drug delivery | Bone tissue engineering, load-bearing applications |
| Examples | Alginate, collagen, polyethylene glycol (PEG) hydrogels | Polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL) |
Introduction to Scaffold Technologies in Biomedical Engineering
Hydrogel scaffolds offer high water content and excellent biocompatibility, closely mimicking the natural extracellular matrix for cell growth in tissue engineering. Polymer scaffolds provide customizable mechanical properties and controlled degradation rates, enabling tailored support for different tissue types. Scaffold technologies leverage these materials to create three-dimensional frameworks that facilitate cell adhesion, proliferation, and differentiation in biomedical engineering applications.
Overview of Hydrogel Scaffolds
Hydrogel scaffolds provide a three-dimensional, water-rich network that closely mimics the natural extracellular matrix, promoting cell attachment and proliferation in tissue engineering. Their high biocompatibility and tunable mechanical properties support controlled drug delivery and tissue regeneration more effectively than traditional polymer scaffolds. Unlike polymer scaffolds, hydrogels offer superior nutrient diffusion and flexibility, making them ideal for soft tissue applications.
Overview of Polymer Scaffolds
Polymer scaffolds are widely used in tissue engineering due to their versatile mechanical properties, biocompatibility, and biodegradability. These scaffolds can be fabricated from natural polymers like collagen and chitosan, or synthetic polymers such as polylactic acid (PLA) and polycaprolactone (PCL), allowing customization for specific tissue regeneration needs. Their porous structure facilitates cell adhesion, proliferation, and nutrient diffusion, making them essential for supporting tissue growth and repair.
Biocompatibility: Hydrogels vs. Polymers
Hydrogel scaffolds exhibit superior biocompatibility compared to conventional polymer scaffolds due to their high water content and tissue-like physical properties, which promote cell adhesion, proliferation, and nutrient diffusion. Polymer scaffolds, while structurally robust, often require surface modifications to reduce cytotoxicity and improve cell interactions. The hydrophilic nature and porosity of hydrogels support enhanced integration with biological tissues, reducing inflammation and rejection risks during regenerative medicine applications.
Mechanical Properties Comparison
Hydrogel scaffolds exhibit lower mechanical strength and stiffness compared to polymer scaffolds, making them more suitable for soft tissue engineering applications where flexibility and biocompatibility are critical. Polymer scaffolds, often composed of materials like PLGA or PCL, provide superior tensile strength and structural integrity, essential for load-bearing tissues such as bone and cartilage. The mechanical properties of polymer scaffolds can be precisely tuned through polymer composition and fabrication techniques, whereas hydrogel scaffolds offer a viscoelastic nature that closely mimics native extracellular matrix mechanics.
Degradation and Bioactivity Differences
Hydrogel scaffolds exhibit rapid degradation rates due to their high water content and enzymatic sensitivity, facilitating timely tissue regeneration, whereas polymer scaffolds typically degrade more slowly through hydrolysis or oxidation, providing prolonged structural support. Hydrogel scaffolds demonstrate enhanced bioactivity by mimicking extracellular matrix properties, promoting cell adhesion, proliferation, and differentiation, while polymer scaffolds often require surface modifications or incorporation of bioactive molecules to achieve comparable cellular responses. The differential degradation kinetics and intrinsic bioactivity of hydrogel versus polymer scaffolds critically influence their suitability for varied tissue engineering applications.
Cell Interaction and Tissue Integration
Hydrogel scaffolds offer superior cell interaction due to their high water content and biocompatibility, closely mimicking the natural extracellular matrix and promoting cell adhesion, proliferation, and differentiation. Polymer scaffolds, often composed of synthetic materials like polylactic acid (PLA) or polyglycolic acid (PGA), provide robust mechanical strength but may require surface modification to enhance cell attachment and bioactivity. Tissue integration is more effective with hydrogel scaffolds as they facilitate nutrient diffusion and support angiogenesis, while polymer scaffolds typically integrate slower but offer customizable degradation rates to match tissue regeneration timelines.
Applications in Tissue Engineering
Hydrogel scaffolds provide a highly hydrated environment mimicking natural extracellular matrix, promoting cell adhesion, proliferation, and differentiation, making them ideal for soft tissue engineering such as cartilage and skin regeneration. Polymer scaffolds, often composed of biocompatible materials like polylactic acid (PLA) and polycaprolactone (PCL), offer superior mechanical strength and structural support, suitable for bone and load-bearing tissue applications. Both scaffold types are integral to tissue engineering strategies, with hydrogels excelling in cell encapsulation and nutrient diffusion, while polymers ensure durability and shape retention.
Challenges and Limitations
Hydrogel scaffolds often face challenges such as limited mechanical strength and rapid degradation, which can compromise their structural integrity in tissue engineering applications. Polymer scaffolds may exhibit issues including poor biocompatibility and potential toxicity from degradation products, restricting their use in sensitive biological environments. Both scaffold types require precise control over porosity, degradation rates, and cell adhesion properties to optimize tissue regeneration outcomes.
Future Perspectives of Scaffold Innovations
Hydrogel scaffolds offer unparalleled biocompatibility and tunable mechanical properties, making them ideal for mimicking the extracellular matrix in tissue engineering, while polymer scaffolds provide superior structural integrity and customizable degradation rates for long-term support. Future perspectives emphasize integrating smart responsive materials into hydrogel-polymer composites to enable dynamic cell signaling and controlled release of bioactive molecules, enhancing tissue regeneration outcomes. Advances in 3D bioprinting and nanotechnology will accelerate the development of hybrid scaffolds with hierarchical architectures that better replicate native tissue microenvironments and improve clinical translation.
Crosslinking Density
Hydrogel scaffolds exhibit tunable crosslinking density that enhances cellular infiltration and nutrient diffusion compared to polymer scaffolds, which often have higher crosslinking density leading to reduced porosity and limited biocompatibility.
Biodegradability
Hydrogel scaffolds offer superior biodegradability over polymer scaffolds due to their high water content and enzymatic degradation pathways.
Porosity Gradients
Hydrogel scaffolds exhibit superior porosity gradients compared to polymer scaffolds, enhancing cellular infiltration and nutrient diffusion for tissue engineering applications.
Swelling Ratio
Hydrogel scaffolds exhibit a significantly higher swelling ratio compared to polymer scaffolds, enhancing nutrient diffusion and cell proliferation in tissue engineering applications.
Hydrophilicity Contrast
Hydrogel scaffolds exhibit superior hydrophilicity compared to polymer scaffolds, enhancing cell adhesion, nutrient diffusion, and tissue integration in biomedical applications.
Biofunctionalization
Hydrogel scaffolds offer superior biofunctionalization compared to polymer scaffolds due to their enhanced cell adhesion, biocompatibility, and tunable biochemical properties enabling targeted tissue engineering applications.
Mechanical Modulus
Hydrogel scaffolds exhibit lower mechanical modulus compared to polymer scaffolds, making them more suitable for soft tissue engineering applications requiring elasticity and flexibility.
Cell Encapsulation
Hydrogel scaffolds provide superior cell encapsulation due to their high water content and biocompatibility, promoting cell viability and nutrient diffusion more effectively than polymer scaffolds.
In Situ Gelation
Hydrogel scaffolds offer superior in situ gelation capabilities compared to polymer scaffolds, enabling minimally invasive delivery and precise tissue conformity for enhanced regenerative medicine applications.
Surface Topography
Hydrogel scaffolds exhibit superior surface topography with enhanced porosity and biocompatibility compared to polymer scaffolds, promoting improved cell adhesion and proliferation.
hydrogel scaffolds vs polymer scaffolds Infographic
 njnir.com
njnir.com