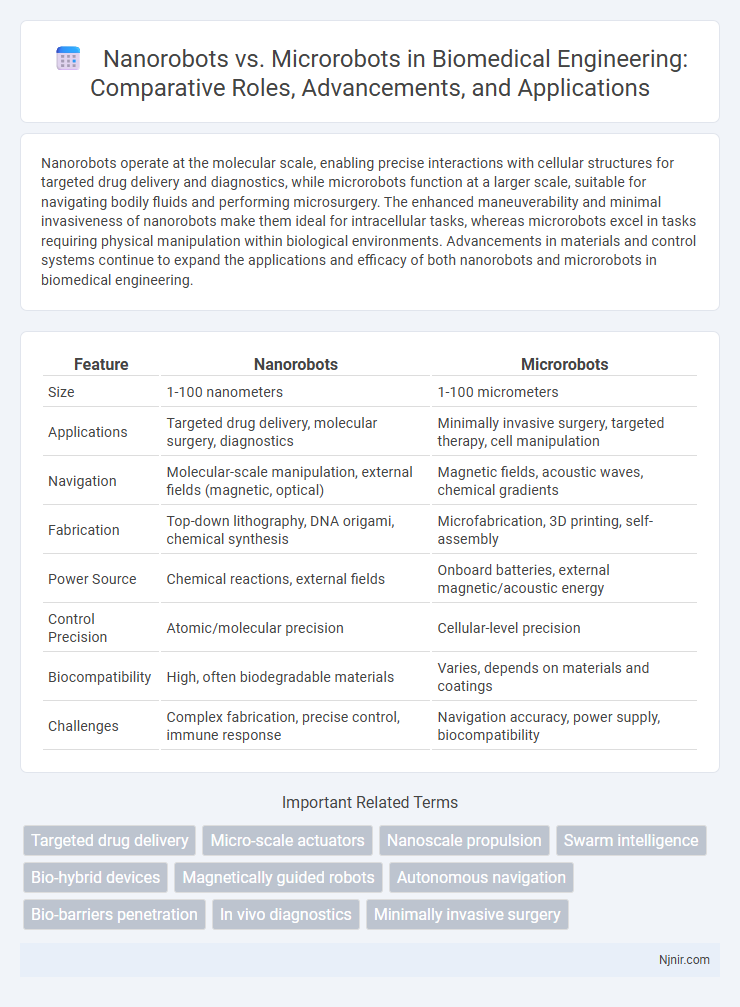
Nanorobots operate at the molecular scale, enabling precise interactions with cellular structures for targeted drug delivery and diagnostics, while microrobots function at a larger scale, suitable for navigating bodily fluids and performing microsurgery. The enhanced maneuverability and minimal invasiveness of nanorobots make them ideal for intracellular tasks, whereas microrobots excel in tasks requiring physical manipulation within biological environments. Advancements in materials and control systems continue to expand the applications and efficacy of both nanorobots and microrobots in biomedical engineering.
Table of Comparison
| Feature | Nanorobots | Microrobots |
|---|---|---|
| Size | 1-100 nanometers | 1-100 micrometers |
| Applications | Targeted drug delivery, molecular surgery, diagnostics | Minimally invasive surgery, targeted therapy, cell manipulation |
| Navigation | Molecular-scale manipulation, external fields (magnetic, optical) | Magnetic fields, acoustic waves, chemical gradients |
| Fabrication | Top-down lithography, DNA origami, chemical synthesis | Microfabrication, 3D printing, self-assembly |
| Power Source | Chemical reactions, external fields | Onboard batteries, external magnetic/acoustic energy |
| Control Precision | Atomic/molecular precision | Cellular-level precision |
| Biocompatibility | High, often biodegradable materials | Varies, depends on materials and coatings |
| Challenges | Complex fabrication, precise control, immune response | Navigation accuracy, power supply, biocompatibility |
Introduction to Nanorobots and Microrobots in Biomedical Engineering
Nanorobots and microrobots are revolutionary technologies in biomedical engineering designed for targeted drug delivery, minimally invasive surgeries, and precise diagnostics. Nanorobots operate at the molecular scale, enabling interaction with cellular structures and facilitating precise therapeutic interventions, while microrobots function at a slightly larger scale, offering enhanced maneuverability within bodily fluids. Their integration into medical applications enhances treatment efficacy, reduces side effects, and opens new possibilities for personalized medicine.
Historical Evolution of Biomedical Nanorobots and Microrobots
Biomedical nanorobots and microrobots have evolved significantly since their conceptual inception in the late 20th century, driven by advances in nanotechnology and microfabrication techniques. Early microrobots emerged in the 1980s with basic mechanical components, while nanorobots began to take shape in the early 2000s, leveraging molecular machinery inspired by biological systems. Continuous improvements in materials science, biocompatibility, and control systems have propelled both fields towards precise medical applications such as targeted drug delivery and minimally invasive surgery.
Structural Differences: Nanorobots vs. Microrobots
Nanorobots typically measure between 1 and 100 nanometers and consist of molecular components arranged to perform specific tasks, often using chemical or biological mechanisms. Microrobots range from 1 micron to several millimeters, featuring more complex mechanical structures such as microelectromechanical systems (MEMS) with actuators, sensors, and onboard power sources. The structural complexity in microrobots allows for greater functional diversity, whereas nanorobots rely on nanoscale materials and molecular assembly for precise interactions at the cellular or molecular level.
Functional Capabilities in Medical Applications
Nanorobots exhibit superior functional capabilities in medical applications due to their ability to operate at the molecular and cellular levels, enabling precise drug delivery, targeted tumor destruction, and real-time cellular diagnostics. Microrobots, though larger, provide effective functionalities such as minimally invasive surgeries, navigation through vascular systems, and tissue manipulation with enhanced mechanical strength. The distinction in scale directly influences their functional capacity, with nanorobots excelling in intracellular tasks while microrobots handle macroscopic therapeutic interventions.
Materials and Fabrication Techniques
Nanorobots are typically fabricated using advanced materials such as carbon nanotubes, graphene, and DNA-based structures, employing techniques like molecular self-assembly and electron beam lithography to achieve precise nanoscale manipulation. Microrobots utilize materials such as polymers, metals, and silicon, with fabrication methods including microelectromechanical systems (MEMS) technology, photolithography, and 3D printing to create functional microscopic devices. The choice of materials and fabrication techniques directly influences the size, functionality, and application scope of nanorobots and microrobots in biomedical and industrial fields.
Precision and Scale: Impacts on Surgical Interventions
Nanorobots operate at the molecular and cellular levels, enabling extreme precision in targeting specific tissues or cells during surgical interventions, which significantly reduces damage to surrounding healthy areas. Microrobots, while larger, provide enhanced mechanical strength and the ability to perform more complex manipulations within a broader anatomical scale, making them suitable for tasks like clearing blockages or delivering drugs within larger vessels. The precision of nanorobots allows for minimally invasive procedures at the nanoscale, whereas microrobots balance precision with functional versatility, impacting surgical outcomes by offering tailored interventions based on the required scale.
Drug Delivery Mechanisms: Nanorobots vs. Microrobots
Nanorobots enable high-precision drug delivery through molecular-level interactions, allowing targeted therapy with minimal side effects by navigating complex biological environments. Microrobots, typically larger, deliver drugs by mechanical transport and controlled release in specific tissues, benefiting from external actuation like magnetic or acoustic fields. The choice between nanorobots and microrobots depends on factors such as drug payload size, target site accessibility, and required control over release kinetics.
Navigation and Control within Biological Environments
Nanorobots navigate biological environments through molecular-scale sensing and propulsion mechanisms, utilizing chemical gradients and magnetic fields for precise control at the cellular level. Microrobots employ micrometer-sized actuators and sensors to achieve targeted motion via magnetic, acoustic, or optical stimuli, allowing them to adapt to complex tissue structures. Both systems rely on real-time feedback control algorithms integrated with imaging techniques such as fluorescence microscopy or MRI for enhanced maneuverability within dynamic and heterogeneous biological matrices.
Current Challenges and Ethical Considerations
Nanorobots face significant challenges in precise navigation within complex biological environments and overcoming immune system responses, while microrobots contend with limited power sources and scalability issues for in vivo applications. Both technologies raise ethical concerns regarding privacy, potential misuse in surveillance, and long-term environmental impacts due to bioaccumulation. Regulatory frameworks are lagging, necessitating comprehensive guidelines to address safety, informed consent, and responsible deployment of nanorobotic and microrobotic systems.
Future Trends and Innovations in Biomedical Robotics
Nanorobots and microrobots are rapidly advancing with innovations emphasizing enhanced precision in drug delivery and minimally invasive surgeries. Future trends include integrating AI-driven control systems for real-time adaptability and biocompatible materials to improve safety and efficacy within the human body. Enhanced swarm robotics and nanoscale fabrication techniques are expected to revolutionize targeted therapies and diagnostics in biomedical applications.
Targeted drug delivery
Nanorobots enable precise targeted drug delivery at the cellular level, surpassing microrobots in navigating complex biological environments and minimizing side effects.
Micro-scale actuators
Micro-scale actuators in microrobots offer precise mechanical control and higher force output compared to nanorobots, enabling enhanced manipulation capabilities in biomedical and industrial applications.
Nanoscale propulsion
Nanorobots utilize nanoscale propulsion mechanisms such as molecular motors and chemical gradients to achieve precise maneuverability, unlike microrobots that rely on microscale actuators and external magnetic or acoustic fields for movement.
Swarm intelligence
Swarm intelligence in nanorobots enables precise, coordinated tasks at molecular scales, surpassing microrobots' capabilities in medical and environmental applications.
Bio-hybrid devices
Bio-hybrid nanorobots, combining synthetic materials with biological components, offer higher precision and biocompatibility compared to microrobots, enabling advanced targeted drug delivery and in vivo diagnostics.
Magnetically guided robots
Magnetically guided nanorobots offer superior precision and navigation at cellular levels compared to larger magnetically guided microrobots, enabling advanced targeted drug delivery and minimally invasive medical procedures.
Autonomous navigation
Nanorobots utilize molecular-scale sensors and AI algorithms for autonomous navigation in complex cellular environments, whereas microrobots rely on microelectromechanical systems (MEMS) and external magnetic fields for guided movement in larger biological and industrial settings.
Bio-barriers penetration
Nanorobots demonstrate superior bio-barrier penetration compared to microrobots due to their smaller size, enhanced maneuverability, and ability to navigate complex biological environments at the cellular and molecular levels.
In vivo diagnostics
Nanorobots enable highly precise in vivo diagnostics by navigating cellular environments at the molecular level, whereas microrobots provide broader real-time monitoring within tissues but with less cellular specificity.
Minimally invasive surgery
Nanorobots enhance minimally invasive surgery by enabling targeted drug delivery and precise cellular-level interventions, whereas microrobots improve navigation and manipulation within larger anatomical structures.
Nanorobots vs Microrobots Infographic
 njnir.com
njnir.com