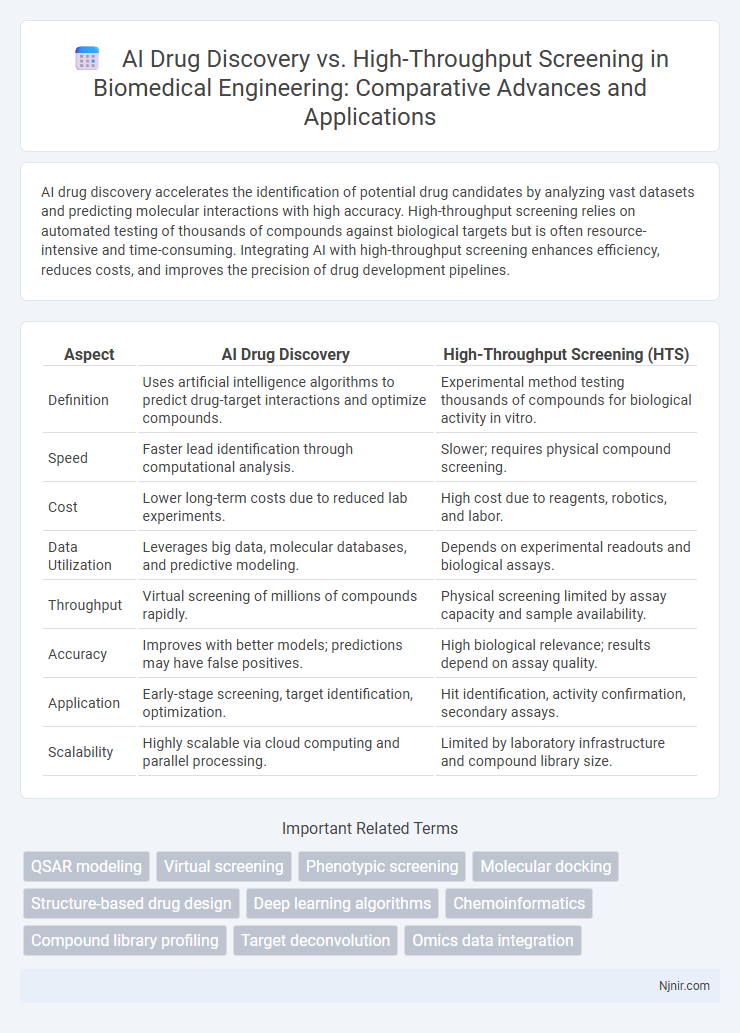
AI drug discovery accelerates the identification of potential drug candidates by analyzing vast datasets and predicting molecular interactions with high accuracy. High-throughput screening relies on automated testing of thousands of compounds against biological targets but is often resource-intensive and time-consuming. Integrating AI with high-throughput screening enhances efficiency, reduces costs, and improves the precision of drug development pipelines.
Table of Comparison
| Aspect | AI Drug Discovery | High-Throughput Screening (HTS) |
|---|---|---|
| Definition | Uses artificial intelligence algorithms to predict drug-target interactions and optimize compounds. | Experimental method testing thousands of compounds for biological activity in vitro. |
| Speed | Faster lead identification through computational analysis. | Slower; requires physical compound screening. |
| Cost | Lower long-term costs due to reduced lab experiments. | High cost due to reagents, robotics, and labor. |
| Data Utilization | Leverages big data, molecular databases, and predictive modeling. | Depends on experimental readouts and biological assays. |
| Throughput | Virtual screening of millions of compounds rapidly. | Physical screening limited by assay capacity and sample availability. |
| Accuracy | Improves with better models; predictions may have false positives. | High biological relevance; results depend on assay quality. |
| Application | Early-stage screening, target identification, optimization. | Hit identification, activity confirmation, secondary assays. |
| Scalability | Highly scalable via cloud computing and parallel processing. | Limited by laboratory infrastructure and compound library size. |
Introduction to AI Drug Discovery and High-Throughput Screening
AI drug discovery leverages machine learning algorithms and vast biomedical datasets to predict molecular interactions, accelerating the identification of potential drug candidates with higher precision. High-throughput screening (HTS) involves automated testing of thousands to millions of chemical compounds against biological targets to identify active compounds rapidly. While HTS generates extensive experimental data, AI integrates this data with computational modeling to enhance drug design efficiency and reduce time and cost in the drug development pipeline.
Evolution of Drug Discovery Methods
AI drug discovery leverages machine learning algorithms to analyze vast biological datasets, accelerating target identification and lead compound optimization compared to traditional high-throughput screening (HTS) that relies on large-scale experimental assays. While HTS systematically tests thousands to millions of compounds against biological targets, AI enhances prediction accuracy and reduces costs by simulating molecular interactions and optimizing candidate selection. The evolution from HTS to AI-driven approaches marks a shift towards integrating computational intelligence with experimental techniques, improving efficiency and success rates in modern drug development pipelines.
Core Principles of AI in Drug Discovery
AI drug discovery leverages machine learning algorithms and deep neural networks to analyze vast biological datasets, identifying novel drug candidates with higher precision compared to traditional high-throughput screening (HTS). By integrating predictive modeling, molecular docking simulations, and virtual screening, AI streamlines the identification of promising compounds from millions of possibilities, reducing time and cost significantly. Core principles of AI in drug discovery include data-driven learning, pattern recognition, and automation, which collectively enhance target identification, lead optimization, and efficacy prediction beyond the capabilities of conventional HTS methods.
High-Throughput Screening: Techniques and Applications
High-throughput screening (HTS) employs automated techniques to rapidly test thousands to millions of compounds for biological activity, significantly accelerating early-stage drug discovery. Techniques in HTS include fluorescence-based assays, mass spectrometry, and cell-based assays, allowing for diverse target interactions and functional readouts. Applications of HTS span lead identification, toxicity profiling, and pathway analysis, making it essential for generating large datasets that inform AI-driven drug discovery models.
Comparative Efficiency: AI vs High-Throughput Screening
AI drug discovery accelerates the identification of potential drug candidates by analyzing vast datasets and predicting molecular interactions with higher precision than high-throughput screening (HTS). While HTS tests thousands of compounds experimentally, AI models reduce time and cost by virtually screening millions of compounds and optimizing lead selection through machine learning algorithms. This computational approach enhances efficiency by minimizing resource-intensive laboratory experiments and focusing efforts on the most promising drug candidates identified via predictive analytics.
Data Integration and Management in Both Approaches
AI drug discovery leverages advanced algorithms to integrate diverse datasets, such as genomics, proteomics, and chemical libraries, enhancing data management through automated processing and real-time analytics. High-throughput screening (HTS) relies on extensive experimental data generation, requiring robust database systems to organize assay results and chemical compound information efficiently. Both approaches depend on effective data integration platforms to correlate biological activity with molecular structures, but AI drug discovery offers superior scalability and insight generation through machine learning-driven data interpretation.
Cost, Speed, and Scalability Analysis
AI drug discovery significantly reduces costs by minimizing the need for extensive physical experiments, offering a more efficient allocation of resources compared to high-throughput screening (HTS), which involves expensive reagents and lab infrastructure. AI algorithms accelerate the drug discovery process by rapidly analyzing vast datasets and predicting molecular interactions, whereas HTS relies on time-consuming experimental assays that limit speed and throughput. Scalability in AI drug discovery is enhanced through cloud computing and advanced data analytics, enabling the processing of millions of compounds simultaneously, whereas HTS scalability is constrained by laboratory capacity and physical automation limitations.
Case Studies: Success Stories and Challenges
AI drug discovery has demonstrated success in accelerating target identification and compound optimization, as seen in projects like Insilico Medicine's rapid identification of novel kinase inhibitors, which reduced development timelines by months. High-throughput screening (HTS) provides extensive empirical data, exemplified by Roche's use of large compound libraries for phenotypic screening, enabling direct activity observation but often requiring costly and time-consuming processes. Challenges in AI include model interpretability and data quality, while HTS struggles with false positives and limited chemical space coverage, making hybrid approaches increasingly valuable.
Regulatory and Ethical Considerations
AI drug discovery accelerates candidate identification while minimizing experimental resource use, presenting unique regulatory challenges in data transparency, algorithm validation, and reproducibility. High-throughput screening (HTS) faces established regulatory frameworks emphasizing assay standardization and toxicity profiling but often involves extensive animal testing, raising ethical concerns. Balancing innovation with patient safety requires evolving guidelines for AI interpretability, data privacy, and minimizing animal use in compliance with ethical standards.
Future Perspectives in Biomedical Engineering
AI drug discovery harnesses machine learning algorithms and big data to predict molecular interactions and optimize drug candidates at a fraction of the time and cost compared to traditional high-throughput screening (HTS). Future perspectives in biomedical engineering emphasize integrating AI with HTS platforms to enhance predictive accuracy, accelerate lead identification, and reduce experimental redundancy. Advances in neural networks, quantum computing, and multi-omics data integration promise to revolutionize personalized medicine and streamline the drug development pipeline.
QSAR modeling
QSAR modeling in AI drug discovery significantly enhances predictive accuracy and cost-efficiency compared to traditional high-throughput screening methods by leveraging computational algorithms to analyze molecular properties and forecast biological activity.
Virtual screening
Virtual screening in AI drug discovery accelerates candidate identification by computationally evaluating millions of compounds, surpassing the time and cost limitations of traditional high-throughput screening methods.
Phenotypic screening
Phenotypic screening in AI drug discovery accelerates target identification by analyzing complex cellular responses faster and more comprehensively than traditional high-throughput screening methods.
Molecular docking
Molecular docking in AI drug discovery enhances prediction accuracy and reduces time compared to traditional high-throughput screening by simulating ligand-receptor interactions at the molecular level.
Structure-based drug design
Structure-based drug design leverages AI algorithms to analyze molecular structures and predict drug-target interactions more efficiently and accurately than traditional high-throughput screening methods.
Deep learning algorithms
Deep learning algorithms enhance AI drug discovery by accurately predicting molecular interactions, surpassing the speed and efficiency of traditional high-throughput screening methods.
Chemoinformatics
Chemoinformatics-driven AI drug discovery accelerates hit identification and optimization with predictive modeling and data integration, surpassing traditional high-throughput screening in efficiency and cost-effectiveness.
Compound library profiling
AI drug discovery enhances compound library profiling by rapidly identifying bioactive molecules with higher accuracy compared to traditional high-throughput screening methods.
Target deconvolution
AI drug discovery accelerates target deconvolution by integrating multi-omics data and predictive modeling, surpassing high-throughput screening's reliance on empirical binding assays.
Omics data integration
Integrating multi-omics data in AI-driven drug discovery enhances target identification and prediction accuracy far beyond traditional high-throughput screening methods.
AI drug discovery vs high-throughput screening Infographic
 njnir.com
njnir.com