Electroporation and sonoporation are two advanced techniques used to enhance cellular permeability for drug and gene delivery in biomedical engineering. Electroporation utilizes short electrical pulses to create temporary pores in the cell membrane, enabling efficient intracellular delivery, while sonoporation employs ultrasound waves combined with microbubble cavitation to disrupt the membrane non-invasively. The selection between these methods depends on factors such as targeted tissue type, delivery efficiency, and potential cellular damage.
Table of Comparison
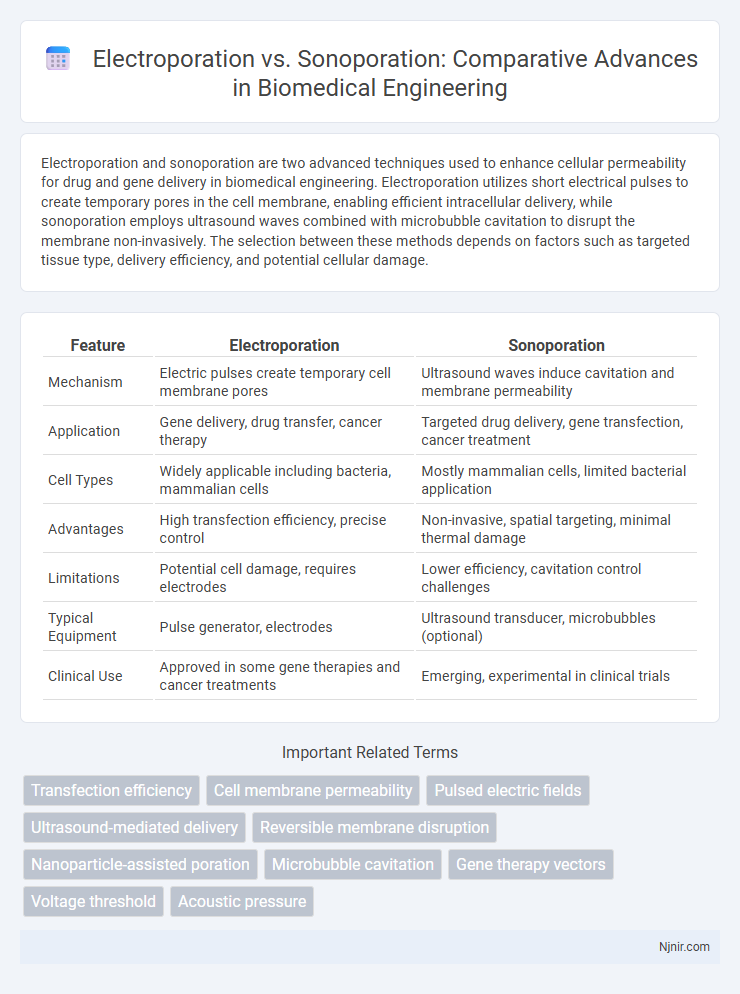
| Feature | Electroporation | Sonoporation |
|---|---|---|
| Mechanism | Electric pulses create temporary cell membrane pores | Ultrasound waves induce cavitation and membrane permeability |
| Application | Gene delivery, drug transfer, cancer therapy | Targeted drug delivery, gene transfection, cancer treatment |
| Cell Types | Widely applicable including bacteria, mammalian cells | Mostly mammalian cells, limited bacterial application |
| Advantages | High transfection efficiency, precise control | Non-invasive, spatial targeting, minimal thermal damage |
| Limitations | Potential cell damage, requires electrodes | Lower efficiency, cavitation control challenges |
| Typical Equipment | Pulse generator, electrodes | Ultrasound transducer, microbubbles (optional) |
| Clinical Use | Approved in some gene therapies and cancer treatments | Emerging, experimental in clinical trials |
Introduction to Electroporation and Sonoporation
Electroporation involves applying short electrical pulses to cells, creating temporary pores in cell membranes to enhance the delivery of molecules like DNA, drugs, or proteins. Sonoporation uses ultrasound waves to induce cavitation effects that transiently disrupt cell membranes, facilitating the entry of therapeutic agents. Both techniques are prominent in targeted drug delivery and gene therapy due to their ability to increase membrane permeability non-invasively.
Fundamental Mechanisms of Electroporation
Electroporation relies on the application of short, high-voltage electric pulses to create transient nanopores within the lipid bilayer of cell membranes, facilitating increased permeability for molecular uptake. These electric pulses induce a reorganization of membrane lipids, leading to the formation of hydrophilic pores that allow molecules such as DNA, drugs, or ions to pass through. The efficiency of electroporation depends on parameters like pulse intensity, duration, and cell type, enabling controlled delivery with minimal cell damage.
Fundamental Mechanisms of Sonoporation
Sonoporation involves the application of ultrasound waves to generate transient pores in cell membranes through acoustic cavitation, enabling enhanced molecular transport. Microbubble oscillation and collapse produce mechanical forces that disrupt lipid bilayers without permanent damage, facilitating targeted drug and gene delivery. This mechanism relies on the careful modulation of ultrasound parameters such as frequency, intensity, and exposure time to optimize membrane permeability while preserving cell viability.
Comparative Efficiency in Drug and Gene Delivery
Electroporation uses electric pulses to create temporary pores in cell membranes, enabling efficient delivery of drugs and genes with high transfection rates, especially in vitro and in tumorous tissues. Sonoporation employs ultrasound waves combined with microbubbles to induce membrane permeability, offering non-invasive delivery with enhanced targeting and reduced cell damage in vivo. Comparative studies demonstrate electroporation achieves higher delivery efficiency for large molecules, while sonoporation provides better spatial control and is more suitable for sensitive tissues and clinical applications.
Applications in Cancer Therapy
Electroporation and sonoporation are advanced techniques for enhancing drug delivery in cancer therapy, with electroporation using electric pulses to increase cell membrane permeability and sonoporation employing ultrasound waves to create temporary pores. Electroporation has demonstrated effectiveness in electrochemotherapy for treating solid tumors such as melanoma and head and neck cancers, allowing targeted drug uptake with minimal systemic toxicity. Sonoporation offers non-invasive delivery of chemotherapeutics and gene therapies, showing promise in improving treatment efficacy for difficult-to-reach tumors like pancreatic and brain cancers.
Safety Profiles and Side Effects
Electroporation and sonoporation both enhance cellular permeability for drug delivery but differ significantly in safety profiles and side effects. Electroporation involves electric pulses that can cause muscle contractions, pain, and localized tissue damage, whereas sonoporation uses ultrasonic waves, often resulting in transient cavitation effects with limited tissue injury and reduced discomfort. Clinical studies indicate that sonoporation presents a safer profile with fewer adverse events, making it preferable for sensitive tissues or repeated treatments.
Technological Advances and Instrumentation
Electroporation technology has advanced with the development of precise pulse generators and electrode designs that enhance cell membrane permeability for targeted drug and gene delivery. Sonoporation instrumentation incorporates high-frequency ultrasound transducers coupled with microbubble contrast agents to induce transient membrane pores while improving targeting accuracy and minimizing tissue damage. Recent innovations in both methods include integration with real-time imaging systems and automation to optimize therapeutic efficacy and control cellular uptake dynamics.
Clinical and Preclinical Studies
Electroporation and sonoporation are prominent techniques for enhancing drug and gene delivery in clinical and preclinical studies, with electroporation using electric pulses to transiently permeabilize cell membranes and sonoporation employing ultrasound waves combined with microbubbles to achieve similar effects. Clinical trials have demonstrated electroporation's effectiveness in delivering chemotherapeutic agents for melanoma and pancreatic cancer, while sonoporation shows promising preclinical results in targeted delivery for brain tumors and cardiovascular diseases. Comparative studies reveal electroporation's advantage in precise spatial control and sonoporation's non-invasive application potential, underscoring their complementary roles in advancing therapeutic delivery systems.
Challenges and Limitations
Electroporation faces challenges such as tissue damage caused by high-voltage pulses and limited penetration depth in dense tissues, which restricts its application in deep-seated tumors. Sonoporation struggles with inconsistent cavitation effects and difficulty in controlling microbubble behavior, leading to variable drug delivery efficiency and potential cell membrane damage. Both techniques require precise optimization of parameters to balance efficacy and safety, and their clinical translation is hindered by these limitations in reproducibility and targeted delivery.
Future Perspectives and Emerging Trends
Electroporation and sonoporation are rapidly advancing in targeted drug delivery and gene therapy, with future perspectives emphasizing enhanced precision and minimal invasiveness. Emerging trends include the integration of nanotechnology and real-time imaging to improve treatment efficacy and cellular transfection rates. These innovations aim to optimize membrane permeability control, enabling personalized medicine and expanding applications in oncology and regenerative medicine.
Transfection efficiency
Electroporation typically achieves higher transfection efficiency compared to sonoporation due to its ability to create uniform and controlled transient pores in the cell membrane, facilitating more effective intracellular delivery of nucleic acids.
Cell membrane permeability
Electroporation increases cell membrane permeability by applying short electrical pulses to create temporary pores, while sonoporation uses ultrasound waves to induce cavitation and disrupt the membrane, enhancing molecular uptake.
Pulsed electric fields
Pulsed electric fields in electroporation create temporary cell membrane pores for enhanced drug and gene delivery, offering precise control compared to sonoporation's ultrasound-induced membrane disruption.
Ultrasound-mediated delivery
Ultrasound-mediated delivery utilizes sonoporation to enhance cellular uptake by temporarily increasing membrane permeability through acoustic cavitation, offering a less invasive and more targeted alternative to electroporation's electric field-based membrane disruption.
Reversible membrane disruption
Electroporation and sonoporation both induce reversible membrane disruption to enhance cellular uptake, with electroporation using electric pulses to create transient pores and sonoporation employing ultrasound-induced cavitation for temporary membrane permeability.
Nanoparticle-assisted poration
Nanoparticle-assisted poration enhances electroporation and sonoporation by improving cell membrane permeability through localized electric field modulation and ultrasonic cavitation effects, respectively, leading to increased efficiency in targeted drug and gene delivery.
Microbubble cavitation
Microbubble cavitation enhances membrane permeability more precisely in sonoporation compared to the electrical pulse method used in electroporation, enabling targeted drug and gene delivery with reduced cellular damage.
Gene therapy vectors
Electroporation enables efficient gene therapy vector delivery by creating temporary cell membrane pores using electrical pulses, while sonoporation uses ultrasound-induced microbubbles to enhance vector uptake, with electroporation generally offering higher transfection efficiency for targeted gene transfer.
Voltage threshold
Electroporation typically requires a voltage threshold of 200-1500 V/cm to create transient pores in cell membranes, whereas sonoporation relies on acoustic pressure thresholds around 0.3-3 MPa to induce membrane permeabilization through ultrasound waves.
Acoustic pressure
Electroporation uses electric fields to increase cell membrane permeability, whereas sonoporation employs varying acoustic pressure from ultrasound waves to create transient pores for molecular delivery.
Electroporation vs Sonoporation Infographic
 njnir.com
njnir.com