Nanorobots offer precise targeting capabilities at the cellular level, allowing for controlled drug delivery and real-time monitoring in biomedical applications. Microbubbles enhance diagnostic imaging and facilitate ultrasound-mediated therapy by improving contrast and enabling localized treatment activation. Comparing both, nanorobots provide active intervention with programmable functions, while microbubbles primarily serve as passive agents to augment imaging and targeted drug release.
Table of Comparison
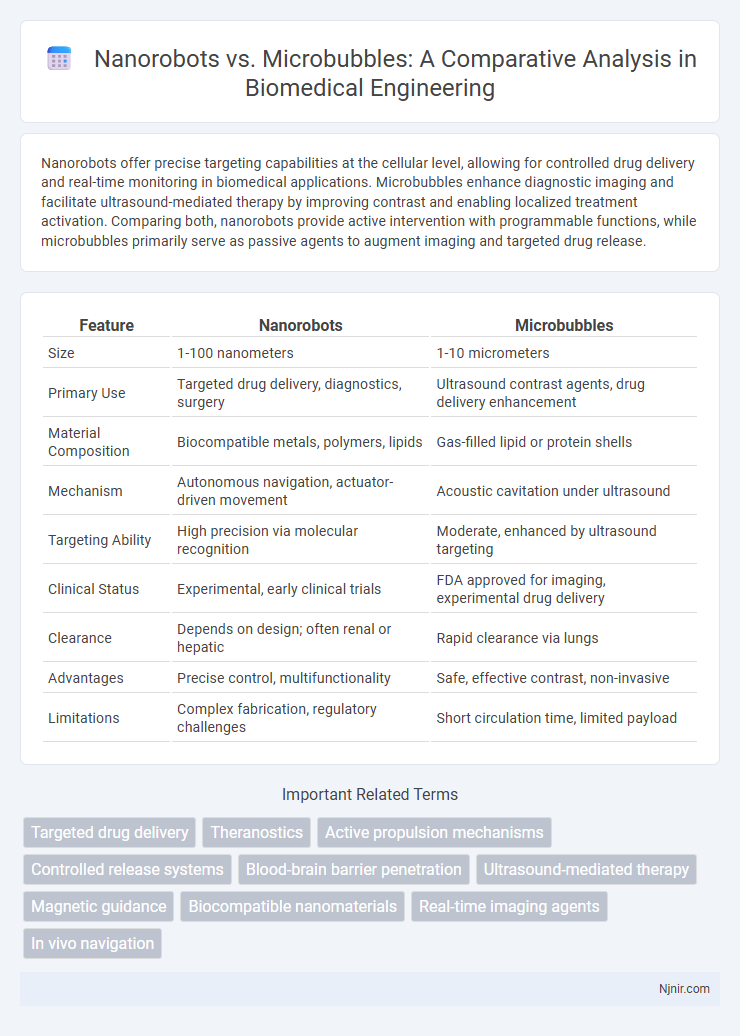
| Feature | Nanorobots | Microbubbles |
|---|---|---|
| Size | 1-100 nanometers | 1-10 micrometers |
| Primary Use | Targeted drug delivery, diagnostics, surgery | Ultrasound contrast agents, drug delivery enhancement |
| Material Composition | Biocompatible metals, polymers, lipids | Gas-filled lipid or protein shells |
| Mechanism | Autonomous navigation, actuator-driven movement | Acoustic cavitation under ultrasound |
| Targeting Ability | High precision via molecular recognition | Moderate, enhanced by ultrasound targeting |
| Clinical Status | Experimental, early clinical trials | FDA approved for imaging, experimental drug delivery |
| Clearance | Depends on design; often renal or hepatic | Rapid clearance via lungs |
| Advantages | Precise control, multifunctionality | Safe, effective contrast, non-invasive |
| Limitations | Complex fabrication, regulatory challenges | Short circulation time, limited payload |
Introduction to Nanorobots and Microbubbles in Biomedical Engineering
Nanorobots and microbubbles represent emerging technologies in biomedical engineering with distinct applications. Nanorobots, designed at the molecular or cellular scale, enable precise drug delivery, targeted diagnostics, and minimally invasive surgeries. Microbubbles serve as contrast agents in ultrasound imaging and can enhance targeted drug delivery through their ability to oscillate and burst under specific acoustic conditions.
Mechanisms of Action: Nanorobots vs Microbubbles
Nanorobots utilize targeted mechanical manipulation and precise navigation at the cellular level to deliver drugs or perform microsurgery, often employing sensors and actuators for real-time response. Microbubbles act primarily through acoustic cavitation, enhancing ultrasound imaging and promoting drug delivery by temporarily disrupting cell membranes under ultrasonic waves. The mechanisms of nanorobots offer active intervention and control, whereas microbubbles rely on passive physical responses to external stimuli.
Design and Fabrication: Nanorobots Compared to Microbubbles
Nanorobots feature intricate nanoscale components engineered through advanced methods like DNA origami and nanoscale 3D printing, enabling precise manipulation at molecular levels. Microbubbles, typically fabricated using ultrasonic emulsification and gas encapsulation within lipid or polymer shells, prioritize stability and acoustic responsiveness for medical imaging and drug delivery. The design of nanorobots emphasizes autonomous functionality and programmable tasks at cellular scales, contrasting with microbubbles' simpler structure optimized for contrast enhancement and targeted therapy.
Drug Delivery Applications: Efficiency and Precision
Nanorobots exhibit superior efficiency in drug delivery applications due to their ability to navigate complex biological environments with high precision, enabling targeted therapy at the cellular and molecular levels. Microbubbles enhance drug delivery primarily through ultrasound-triggered release, which improves localized drug concentration but lacks the autonomous navigation capabilities of nanorobots. Comparative studies highlight that nanorobots achieve greater precision and sustained delivery, while microbubbles offer non-invasive activation methods suitable for short-term treatments.
Targeting Strategies: Navigation and Localization
Nanorobots employ advanced navigation mechanisms such as chemotaxis, magnetic guidance, and ultrasound propulsion to achieve precise cellular targeting, optimizing drug delivery at the molecular level. Microbubbles rely primarily on acoustic targeting through ultrasound waves, enhancing localization by exploiting their resonance properties for site-specific therapeutic and diagnostic applications. Integrating imaging modalities like MRI or ultrasound biomicroscopy further refines the spatial accuracy of both nanorobots and microbubbles, enabling real-time tracking and improved targeting efficacy in vivo.
Diagnostic Capabilities: Imaging and Sensing
Nanorobots offer enhanced diagnostic capabilities through precise targeting and real-time imaging at the cellular level, enabling detailed sensing of biochemical markers. Microbubbles excel in ultrasound imaging by providing strong acoustic contrast, facilitating improved visualization of blood flow and tissue perfusion. Combining nanorobots' nanoscale navigation with microbubbles' ultrasound signal amplification significantly advances non-invasive medical diagnostics.
Biocompatibility and Safety Concerns
Nanorobots demonstrate superior biocompatibility due to their precisely engineered surfaces that minimize immune response and cytotoxicity, whereas microbubbles often face challenges with potential immune activation and embolism risks. Safety concerns with nanorobots primarily revolve around long-term biodegradability and controlled clearance from the body, while microbubbles carry risks of vascular obstruction and cavitation-induced tissue damage. Advances in polymer coatings and targeted delivery systems are crucial for enhancing the safety profiles of both nanorobots and microbubbles in clinical applications.
Therapeutic Potential: Cancer, Cardiovascular, and Neurological Disorders
Nanorobots offer precise targeting and controlled drug delivery to cancer cells, cardiovascular tissues, and neurological regions, enhancing therapeutic outcomes while minimizing side effects. Microbubbles facilitate targeted drug and gene delivery through ultrasound-mediated cavitation, improving treatment efficacy in tumor ablation, plaque stabilization, and blood-brain barrier penetration. Both modalities demonstrate significant potential in personalized medicine, combining diagnostic imaging and localized therapy for complex diseases.
Current Challenges and Technological Limitations
Nanorobots face significant challenges in precise navigation and biocompatibility within complex biological environments, limiting their clinical applications despite advances in targeted drug delivery. Microbubbles are constrained by short circulation times and instability under physiological conditions, restricting their efficacy in ultrasound imaging and therapy. Both technologies require improved control mechanisms and enhancement in stability to realize their full potential in medical diagnostics and treatment.
Future Prospects and Emerging Trends in Nanorobots and Microbubbles
Nanorobots and microbubbles are advancing rapidly in targeted drug delivery and diagnostic imaging, with nanorobots offering precision at the cellular level and programmable functions for personalized medicine. Emerging trends include the integration of artificial intelligence for autonomous navigation and enhanced biocompatibility to minimize immune response, while microbubbles are evolving with novel contrast agents and ultrasound-triggered drug release systems. Future prospects highlight hybrid systems combining nanorobots and microbubbles to synergistically improve therapeutic efficacy and real-time monitoring in clinical applications.
Targeted drug delivery
Nanorobots offer precise targeted drug delivery by navigating cellular environments autonomously, whereas microbubbles enhance targeted delivery through ultrasound-triggered payload release and improved vascular permeability.
Theranostics
Nanorobots enable precise, targeted drug delivery and real-time imaging in theranostics, while microbubbles primarily enhance ultrasound contrast and facilitate localized drug release for improved diagnostic and therapeutic outcomes.
Active propulsion mechanisms
Nanorobots utilize advanced active propulsion mechanisms like magnetic and acoustic actuation for precise navigation, whereas microbubbles primarily rely on acoustic radiation forces and gas diffusion-driven propulsion in biomedical applications.
Controlled release systems
Nanorobots provide precise controlled release systems by targeting specific cells with programmable mechanisms, unlike microbubbles which rely on ultrasound-triggered release primarily for localized drug delivery.
Blood-brain barrier penetration
Nanorobots exhibit superior precision and controlled penetration of the blood-brain barrier compared to microbubbles, enabling targeted drug delivery with enhanced efficacy and reduced systemic side effects.
Ultrasound-mediated therapy
Nanorobots offer precise targeting and controlled drug delivery, whereas microbubbles enhance ultrasound-mediated therapy by improving contrast and facilitating localized drug release through cavitation effects.
Magnetic guidance
Nanorobots offer superior magnetic guidance capabilities compared to microbubbles, enabling precise navigation and targeted drug delivery at the cellular level.
Biocompatible nanomaterials
Biocompatible nanomaterials in nanorobots offer targeted drug delivery with precise control and minimal immune response, whereas microbubbles primarily enhance ultrasound imaging and drug delivery but have limited functionalization and targeting capabilities.
Real-time imaging agents
Nanorobots provide targeted delivery and real-time imaging capabilities with high precision, while microbubbles offer enhanced ultrasound contrast and dynamic vascular imaging for real-time visualization in medical diagnostics.
In vivo navigation
Nanorobots enable precise in vivo navigation by actively targeting tissues at the cellular level, whereas microbubbles primarily enhance ultrasound imaging through passive navigation and contrast enhancement.
Nanorobots vs Microbubbles Infographic
 njnir.com
njnir.com