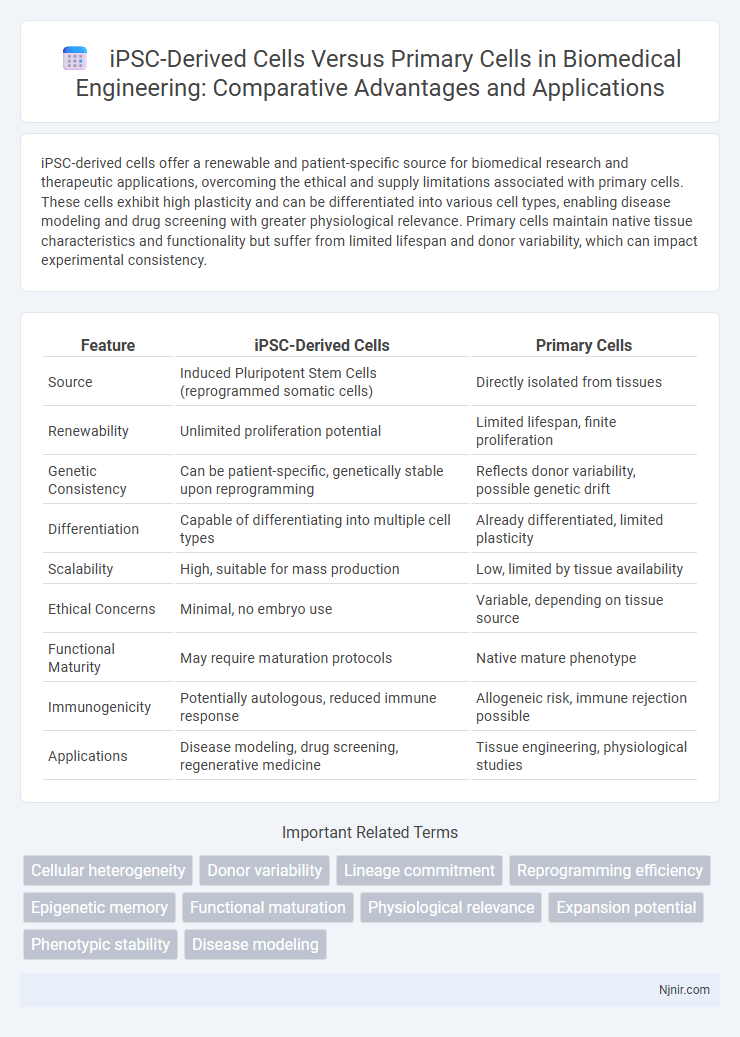
iPSC-derived cells offer a renewable and patient-specific source for biomedical research and therapeutic applications, overcoming the ethical and supply limitations associated with primary cells. These cells exhibit high plasticity and can be differentiated into various cell types, enabling disease modeling and drug screening with greater physiological relevance. Primary cells maintain native tissue characteristics and functionality but suffer from limited lifespan and donor variability, which can impact experimental consistency.
Table of Comparison
| Feature | iPSC-Derived Cells | Primary Cells |
|---|---|---|
| Source | Induced Pluripotent Stem Cells (reprogrammed somatic cells) | Directly isolated from tissues |
| Renewability | Unlimited proliferation potential | Limited lifespan, finite proliferation |
| Genetic Consistency | Can be patient-specific, genetically stable upon reprogramming | Reflects donor variability, possible genetic drift |
| Differentiation | Capable of differentiating into multiple cell types | Already differentiated, limited plasticity |
| Scalability | High, suitable for mass production | Low, limited by tissue availability |
| Ethical Concerns | Minimal, no embryo use | Variable, depending on tissue source |
| Functional Maturity | May require maturation protocols | Native mature phenotype |
| Immunogenicity | Potentially autologous, reduced immune response | Allogeneic risk, immune rejection possible |
| Applications | Disease modeling, drug screening, regenerative medicine | Tissue engineering, physiological studies |
Introduction to iPSC-Derived and Primary Cells in Biomedical Engineering
Induced pluripotent stem cell (iPSC)-derived cells offer a renewable and patient-specific source for biomedical engineering, enabling disease modeling, drug screening, and regenerative therapies with high genetic relevance. Primary cells, isolated directly from tissues, maintain native phenotypes and physiological functions but face limited expansion capacity and donor variability challenges. The integration of iPSC-derived cells with primary cell data enhances the development of personalized and functional tissue models in biomedical research.
Sources and Isolation Techniques
iPSC-derived cells originate from adult somatic cells reprogrammed into pluripotent stem cells, allowing differentiation into various cell types, while primary cells are directly isolated from tissues using enzymatic digestion or mechanical methods. Induced pluripotent stem cells require reprogramming techniques such as viral transduction or non-integrative methods to induce pluripotency, whereas primary cells are obtained through tissue-specific isolation protocols that preserve native characteristics. The source flexibility of iPSCs enables generation of diverse cell types from limited initial samples, contrasting with primary cells' dependency on fresh tissue and more variable isolation success rates.
Cellular Characterization and Identity
iPSC-derived cells exhibit dynamic cellular characterization reflecting pluripotency markers such as OCT4, SOX2, and NANOG during reprogramming, transitioning to specialized lineage-specific markers upon differentiation, while primary cells maintain stable expression of their inherent phenotypic markers. Cellular identity in iPSC-derived cells requires rigorous validation through genomic, epigenomic, and proteomic profiling to confirm lineage fidelity and functional equivalence to primary cells, which inherently possess native cellular phenotypes and in vivo functionality. Variability in iPSC-derived cell populations necessitates standardized characterization protocols to ensure reproducibility and comparability with primary cells for translational research and therapeutic applications.
Genetic Stability and Variability
iPSC-derived cells exhibit increased genetic variability compared to primary cells due to reprogramming-induced mutations and culture adaptation over passages, which can impact experimental reproducibility. Primary cells maintain higher genetic stability as they are directly isolated from tissues, preserving native genomic integrity but with limited proliferative capacity. Understanding these differences is critical for applications in disease modeling and regenerative medicine where genetic fidelity influences functional outcomes.
Functional Performance in Experimental Models
iPSC-derived cells exhibit remarkable functional performance in experimental models, often mirroring the physiological characteristics of primary cells while offering scalability and genetic manipulability advantages. Primary cells retain native tissue-specific functionalities with higher fidelity and reduced phenotypic drift but are limited by donor variability and finite lifespan ex vivo. Comparative studies demonstrate iPSC-derived cardiomyocytes and neurons can recapitulate disease phenotypes and electrophysiological properties, though primary cells still outperform in mature functional assays and long-term stability.
Disease Modeling: iPSC-derived vs Primary Cells
iPSC-derived cells offer a significant advantage for disease modeling by providing patient-specific genetic backgrounds and the ability to generate diverse cell types from a single donor, enabling precise study of genetic diseases and personalized medicine. Primary cells, while retaining native physiological properties, are limited by donor variability, finite lifespan, and difficulty in obtaining diseased tissue relevant to certain conditions. iPSC-derived cells enable high-throughput screening and mechanistic studies of complex diseases, making them a versatile tool compared to the often less accessible and heterogenous primary cells.
Applications in Drug Screening and Toxicity Testing
iPSC-derived cells offer a renewable and genetically diverse source for drug screening and toxicity testing, enabling the modeling of patient-specific responses and rare genetic disorders. Primary cells retain native physiological properties and metabolic profiles, providing highly relevant context for acute toxicity assessment and pharmacodynamics studies. Combining iPSC-derived and primary cells enhances predictive accuracy in preclinical drug evaluation by capturing both genetic variability and authentic tissue-specific behavior.
Clinical Translation Potential and Safety Concerns
iPSC-derived cells offer significant clinical translation potential due to their ability to provide patient-specific, renewable sources of various cell types without the ethical concerns associated with embryonic stem cells. Primary cells, while naturally sourced and typically exhibiting mature phenotypes, often face limitations in scalability and donor variability, impacting their reproducibility in therapies. Safety concerns for iPSC-derived cells include the risks of genetic abnormalities and tumorigenicity, necessitating rigorous screening and quality control, whereas primary cells generally present lower oncogenic risks but may carry donor-specific pathogens or age-related dysfunctions.
Limitations and Challenges
iPSC-derived cells face limitations such as incomplete maturation and phenotypic variability, which can impact their functional fidelity compared to primary cells. Challenges include potential genetic and epigenetic abnormalities acquired during reprogramming and differentiation processes. Primary cells, while more physiologically relevant, suffer from limited availability, donor variability, and reduced proliferative capacity, complicating large-scale applications.
Future Perspectives in Cell-Based Biomedical Engineering
iPSC-derived cells offer unparalleled potential for personalized medicine and regenerative therapies due to their ability to differentiate into various cell types while overcoming limitations of donor variability inherent in primary cells. Advances in gene editing and 3D bioprinting technologies are enhancing the scalability and functional maturity of iPSC-derived cells, positioning them as a cornerstone for next-generation tissue engineering and disease modeling. Continued optimization of differentiation protocols and integration with microfluidic systems will drive the future of cell-based biomedical engineering toward more precise, patient-specific therapeutic applications.
Cellular heterogeneity
iPSC-derived cells exhibit greater cellular heterogeneity compared to primary cells due to variable differentiation efficiency and incomplete maturation processes.
Donor variability
iPSC-derived cells offer reduced donor variability compared to primary cells by providing consistent genetic backgrounds for reproducible experimental outcomes.
Lineage commitment
iPSC-derived cells exhibit flexible lineage commitment allowing differentiation into multiple cell types, whereas primary cells demonstrate more restricted lineage commitment reflecting their tissue-specific origins.
Reprogramming efficiency
iPSC-derived cells exhibit variable reprogramming efficiency influenced by donor cell type and methodology, often lower than the natural functionality and stability observed in primary cells.
Epigenetic memory
iPSC-derived cells retain partial epigenetic memory from their donor somatic cells, influencing differentiation potential, whereas primary cells maintain native epigenetic profiles crucial for specific tissue functions.
Functional maturation
iPSC-derived cells exhibit improved functional maturation with enhanced electrophysiological properties and metabolic activity compared to primary cells, offering greater potential for disease modeling and regenerative medicine.
Physiological relevance
iPSC-derived cells offer enhanced physiological relevance by providing patient-specific, genetically accurate models that better mimic native tissue functions compared to primary cells, which often suffer from limited availability and donor variability.
Expansion potential
iPSC-derived cells exhibit significantly higher expansion potential than primary cells, enabling large-scale production for research and therapeutic applications.
Phenotypic stability
iPSC-derived cells exhibit variable phenotypic stability compared to primary cells, often requiring optimized culture conditions to maintain consistent functional characteristics over extended passages.
Disease modeling
iPSC-derived cells enable precise disease modeling by replicating patient-specific genetic profiles, whereas primary cells offer limited scalability and variability for studying chronic disease mechanisms.
iPSC-derived cells vs Primary cells Infographic
 njnir.com
njnir.com