Hydrogel scaffolds offer tunable mechanical properties and high biocompatibility, promoting cell proliferation and differentiation in tissue engineering. Decellularized scaffolds retain native extracellular matrix architecture and biochemical cues, enhancing tissue integration and reducing immune response. Comparing both, hydrogels provide customizable environments, while decellularized scaffolds deliver naturally preserved microenvironments essential for functional tissue regeneration.
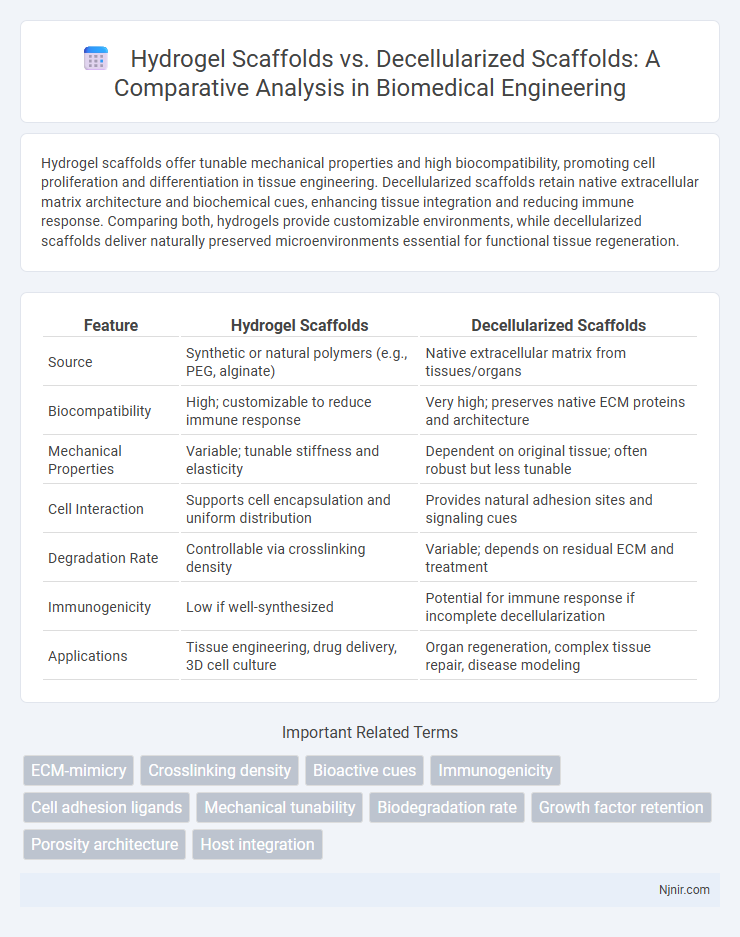
Table of Comparison
| Feature | Hydrogel Scaffolds | Decellularized Scaffolds |
|---|---|---|
| Source | Synthetic or natural polymers (e.g., PEG, alginate) | Native extracellular matrix from tissues/organs |
| Biocompatibility | High; customizable to reduce immune response | Very high; preserves native ECM proteins and architecture |
| Mechanical Properties | Variable; tunable stiffness and elasticity | Dependent on original tissue; often robust but less tunable |
| Cell Interaction | Supports cell encapsulation and uniform distribution | Provides natural adhesion sites and signaling cues |
| Degradation Rate | Controllable via crosslinking density | Variable; depends on residual ECM and treatment |
| Immunogenicity | Low if well-synthesized | Potential for immune response if incomplete decellularization |
| Applications | Tissue engineering, drug delivery, 3D cell culture | Organ regeneration, complex tissue repair, disease modeling |
Introduction to Scaffold Technologies in Biomedical Engineering
Hydrogel scaffolds consist of hydrophilic polymer networks that mimic the extracellular matrix, providing a highly hydrated environment favorable for cell proliferation and tissue regeneration. Decellularized scaffolds are derived from native tissues with cellular components removed, preserving the complex extracellular matrix architecture and biochemical cues essential for tissue-specific regeneration. Both scaffold types play crucial roles in biomedical engineering by offering distinct advantages in mechanical properties, biocompatibility, and bioactivity for tissue engineering applications.
Overview of Hydrogel Scaffolds
Hydrogel scaffolds are three-dimensional, hydrophilic polymer networks that provide a highly porous and biocompatible environment for cell growth and tissue regeneration. These scaffolds mimic the extracellular matrix by maintaining high water content, facilitating nutrient diffusion, and supporting cell adhesion and proliferation. Commonly composed of natural polymers like collagen or synthetic materials such as PEG, hydrogel scaffolds offer tunable mechanical properties and degradation rates tailored to specific tissue engineering applications.
Fundamentals of Decellularized Scaffolds
Decellularized scaffolds are derived from native tissues by removing cellular components while preserving the extracellular matrix (ECM), crucial for maintaining structural integrity and biochemical cues for cell attachment and differentiation. Unlike hydrogel scaffolds, which are synthetic or semi-synthetic polymer networks designed to mimic ECM properties, decellularized scaffolds retain the natural architecture, mechanical strength, and bioactive molecules that support tissue regeneration. The fundamental advantage of decellularized scaffolds lies in their ability to provide an intrinsic microenvironment conducive to tissue remodeling and reduced immunogenicity.
Fabrication Methods: Hydrogel vs. Decellularized Scaffolds
Hydrogel scaffolds are fabricated through processes like photopolymerization, ionic crosslinking, and temperature-induced gelation, allowing precise control over their physical properties and biocompatibility. Decellularized scaffolds are produced by removing cellular components from native tissues using chemical, enzymatic, or mechanical methods, preserving the extracellular matrix structure. The choice between hydrogel and decellularized scaffold fabrication depends on desired matrix complexity, tissue specificity, and regenerative application.
Biocompatibility and Bioactivity Comparison
Hydrogel scaffolds exhibit superior biocompatibility due to their high water content and tunable properties that mimic native extracellular matrix, promoting cell adhesion and proliferation. Decellularized scaffolds retain native tissue architecture and bioactive cues, enhancing bioactivity by preserving natural growth factors and signaling molecules critical for tissue regeneration. While hydrogel scaffolds offer customizable environments for cell encapsulation, decellularized scaffolds provide intrinsic bioactivity that supports cellular differentiation and functional tissue integration.
Mechanical Properties and Structural Integrity
Hydrogel scaffolds exhibit tunable mechanical properties with high water content, providing a soft and flexible matrix that mimics native tissue elasticity but often lacks sufficient structural integrity for load-bearing applications. Decellularized scaffolds maintain the native extracellular matrix architecture, offering superior mechanical strength and structural integrity essential for supporting tissue regeneration under physiological stresses. The choice between hydrogel and decellularized scaffolds depends on the targeted tissue's mechanical demands, with hydrogels favored for soft tissues and decellularized scaffolds preferred for tissues requiring robust mechanical support.
Cellular Interactions and Tissue Regeneration Potential
Hydrogel scaffolds provide a highly hydrated, three-dimensional environment that mimics the extracellular matrix, promoting cell adhesion, proliferation, and migration through tailored biochemical cues and tunable mechanical properties. Decellularized scaffolds retain native tissue architecture and complex extracellular matrix proteins, offering natural binding sites that enhance cellular attachment and facilitate tissue-specific regeneration. Both scaffold types support tissue regeneration, but hydrogel scaffolds allow greater customization for controlled release of growth factors, whereas decellularized scaffolds provide intrinsic bioactive signals critical for functional tissue remodeling.
Applications in Regenerative Medicine
Hydrogel scaffolds provide a highly tunable microenvironment that supports cell proliferation, differentiation, and tissue regeneration, making them ideal for applications such as cartilage repair, wound healing, and drug delivery in regenerative medicine. Decellularized scaffolds, derived from native extracellular matrices, retain the natural architecture and biochemical cues essential for tissue-specific regeneration, particularly effective in organ transplantation, cardiac tissue engineering, and skin regeneration. Both scaffold types play crucial roles in regenerative medicine by promoting functional tissue integration and enhancing the body's innate healing processes.
Challenges and Limitations of Both Scaffold Types
Hydrogel scaffolds often face challenges such as limited mechanical strength and potential rapid degradation, which can hinder their application in load-bearing tissues. Decellularized scaffolds present limitations including batch-to-batch variability and the risk of residual immunogenic molecules that may trigger immune responses. Both scaffold types require precise optimization to balance biocompatibility, structural integrity, and functional integration in tissue engineering.
Future Perspectives and Emerging Trends
Hydrogel scaffolds are undergoing rapid advancements in tunable mechanical properties and bioactive molecule incorporation, promoting precise cell differentiation and tissue regeneration. Decellularized scaffolds are increasingly integrated with genetic engineering and nanotechnology to enhance cell compatibility and vascularization, addressing limitations in structural integrity. Emerging trends highlight hybrid scaffold systems combining hydrogels and decellularized matrices to optimize regenerative outcomes and accelerate clinical translation.
ECM-mimicry
Hydrogel scaffolds provide customizable biochemical and mechanical cues closely mimicking native extracellular matrix (ECM) properties, whereas decellularized scaffolds retain the complex, natural ECM architecture and biochemical composition essential for tissue regeneration.
Crosslinking density
Hydrogel scaffolds exhibit tunable crosslinking density for controlled mechanical properties, whereas decellularized scaffolds possess fixed crosslinking structures derived from native extracellular matrix components.
Bioactive cues
Hydrogel scaffolds provide customizable bioactive cues through encapsulated growth factors and peptides, whereas decellularized scaffolds offer native extracellular matrix-derived signals that enhance cell adhesion and tissue-specific regeneration.
Immunogenicity
Hydrogel scaffolds exhibit lower immunogenicity compared to decellularized scaffolds due to their synthetic polymer composition and reduced presence of donor cellular antigens.
Cell adhesion ligands
Hydrogel scaffolds offer tunable cell adhesion ligands that enhance cell attachment and proliferation, whereas decellularized scaffolds provide native extracellular matrix-derived ligands for more physiological cell adhesion and signaling.
Mechanical tunability
Hydrogel scaffolds offer superior mechanical tunability with adjustable stiffness and elasticity through polymer composition modifications, whereas decellularized scaffolds provide native extracellular matrix architecture but limited mechanical customization.
Biodegradation rate
Hydrogel scaffolds exhibit a faster biodegradation rate compared to decellularized scaffolds, which degrade more slowly due to their preserved extracellular matrix composition and complex structural integrity.
Growth factor retention
Hydrogel scaffolds exhibit superior growth factor retention compared to decellularized scaffolds due to their tunable polymer networks and enhanced biomimetic properties.
Porosity architecture
Hydrogel scaffolds offer highly tunable porosity architectures enabling controlled cell infiltration and nutrient diffusion, whereas decellularized scaffolds preserve native extracellular matrix porosity that supports natural tissue regeneration.
Host integration
Hydrogel scaffolds enhance host integration through improved cell infiltration and angiogenesis, whereas decellularized scaffolds provide natural extracellular matrix cues that promote tissue-specific remodeling and immune compatibility.
Hydrogel scaffolds vs Decellularized scaffolds Infographic
 njnir.com
njnir.com