Point-of-care devices deliver rapid, on-site diagnostic results, enhancing timely clinical decision-making and patient management. These portable systems reduce the need for sample transportation, minimizing delays and potential sample degradation common in central lab diagnostics. While central labs offer comprehensive testing with higher accuracy, point-of-care technologies provide critical, immediate data that support early diagnosis and treatment in various healthcare settings.
Table of Comparison
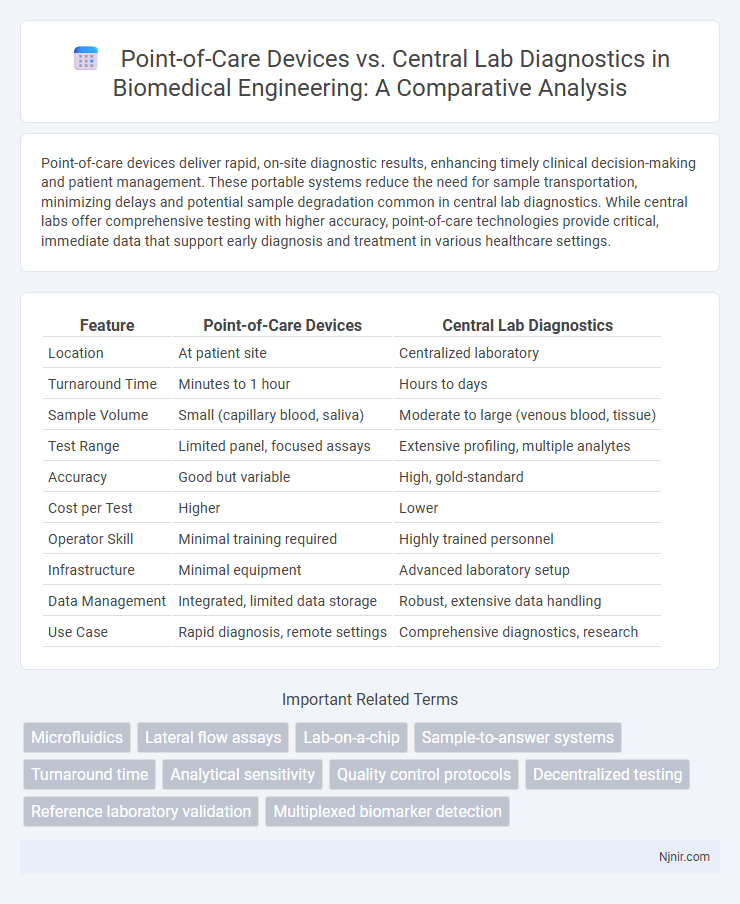
| Feature | Point-of-Care Devices | Central Lab Diagnostics |
|---|---|---|
| Location | At patient site | Centralized laboratory |
| Turnaround Time | Minutes to 1 hour | Hours to days |
| Sample Volume | Small (capillary blood, saliva) | Moderate to large (venous blood, tissue) |
| Test Range | Limited panel, focused assays | Extensive profiling, multiple analytes |
| Accuracy | Good but variable | High, gold-standard |
| Cost per Test | Higher | Lower |
| Operator Skill | Minimal training required | Highly trained personnel |
| Infrastructure | Minimal equipment | Advanced laboratory setup |
| Data Management | Integrated, limited data storage | Robust, extensive data handling |
| Use Case | Rapid diagnosis, remote settings | Comprehensive diagnostics, research |
Introduction to Point-of-Care Devices and Central Lab Diagnostics
Point-of-care devices provide rapid, on-site diagnostic testing, enabling immediate clinical decision-making by delivering results within minutes. In contrast, central lab diagnostics involve comprehensive, high-throughput testing conducted in specialized laboratories with advanced equipment and trained personnel, ensuring higher accuracy and broader test panels. The integration of these diagnostic approaches improves patient outcomes by balancing speed and precision in medical testing.
Technological Advances in Point-of-Care Testing
Technological advances in point-of-care testing have revolutionized rapid diagnostics by integrating microfluidics, biosensors, and smartphone connectivity, enabling immediate results and improved patient management outside central labs. These devices leverage nanotechnology and AI algorithms to enhance sensitivity and specificity, rivaling traditional laboratory accuracy while offering portability and real-time data transmission. Innovations such as multiplexed assays and wearable sensors continue to expand the diagnostic capabilities at the point of care, reducing turnaround times and healthcare costs compared to conventional central laboratory diagnostics.
Workflow Efficiency: Point-of-Care vs Central Laboratory
Point-of-care devices significantly enhance workflow efficiency by providing rapid diagnostic results directly at the patient's location, reducing turnaround time from hours to minutes. Central laboratory diagnostics, while offering higher throughput and advanced testing capabilities, often involve complex sample transportation and processing steps that delay result availability. The streamlined workflow of point-of-care testing minimizes bottlenecks in clinical decision-making, improving patient management and reducing hospital stay durations.
Diagnostic Accuracy and Reliability Comparison
Point-of-care devices offer rapid results with moderate diagnostic accuracy, making them ideal for immediate clinical decisions but often less reliable than central lab diagnostics. Central lab diagnostics utilize advanced, automated equipment and stringent quality controls, providing higher accuracy and consistency in test results. Studies show central labs typically achieve greater sensitivity and specificity, essential for confirmatory diagnoses and complex analyses.
Cost Analysis and Economic Impact
Point-of-care devices significantly reduce operational costs by minimizing the need for specialized personnel and expensive laboratory infrastructure compared to central lab diagnostics. These devices enable faster decision-making, leading to decreased patient length of stay and lower overall healthcare expenses. However, central lab diagnostics often provide higher accuracy and throughput, potentially reducing costs associated with repeat testing and misdiagnosis over time.
Turnaround Time: Rapid Results vs Comprehensive Testing
Point-of-care devices deliver rapid results within minutes, significantly reducing turnaround time for immediate clinical decisions and patient management. Central lab diagnostics offer comprehensive testing with higher analytical accuracy and broader test menus, though results typically require hours to days. This balance between speed and depth influences clinical workflows, with point-of-care ideal for urgent scenarios and central labs preferred for detailed diagnoses.
Accessibility and Implementation in Clinical Settings
Point-of-care devices enhance accessibility by providing rapid diagnostic results at or near the patient site, reducing dependence on central lab infrastructure. Their implementation in clinical settings streamlines workflows, enabling immediate decision-making and improving patient outcomes, especially in remote or resource-limited areas. Central lab diagnostics, while highly accurate and comprehensive, often involve longer turnaround times and logistical challenges that can delay treatment initiation.
Quality Control and Regulatory Considerations
Point-of-care (POC) devices require stringent quality control protocols to ensure accuracy and reliability comparable to central lab diagnostics, often incorporating built-in controls and real-time data monitoring to minimize user errors. Regulatory considerations for POC devices involve compliance with agencies like the FDA and CE, focusing on safety, efficacy, and usability for non-laboratory settings, whereas central lab diagnostics must adhere to comprehensive standards such as CLIA and CAP certifications. Both methodologies emphasize traceability and validation of reagents and equipment, but POC devices demand rapid verification cycles to maintain clinical utility outside traditional laboratory environments.
Applications in Resource-Limited and Remote Environments
Point-of-care devices enable rapid diagnostic testing directly at the site of patient care, significantly improving access in resource-limited and remote environments where central lab facilities are unavailable or under-resourced. These portable devices facilitate timely disease detection and management for conditions such as infectious diseases, chronic illnesses, and maternal health monitoring, thereby reducing delays associated with sample transportation to central labs. Central lab diagnostics, while offering comprehensive and highly accurate analyses, often face logistical challenges and longer turnaround times, limiting their effectiveness in urgent care settings and underserved areas.
Future Trends and Innovations in Biomedical Diagnostics
Point-of-care devices are rapidly evolving with advancements in microfluidics, biosensors, and AI integration, enabling faster, more accurate bedside diagnostics compared to traditional central lab methods. Innovations such as wearable biosensors and portable multiplex testing platforms are facilitating real-time monitoring and personalized healthcare outside conventional labs. Future trends emphasize seamless data connectivity, miniaturization, and enhanced sensitivity, bridging the gap between point-of-care testing and central laboratory precision.
Microfluidics
Microfluidic point-of-care devices enable rapid, low-cost, and accurate diagnostic testing directly at the patient site, contrasting with centralized lab diagnostics that require complex infrastructure and longer turnaround times.
Lateral flow assays
Lateral flow assays in point-of-care devices provide rapid, cost-effective diagnostics with portable convenience, contrasting centralized lab diagnostics' higher sensitivity, quantitative analysis, and comprehensive testing capabilities.
Lab-on-a-chip
Lab-on-a-chip technology revolutionizes diagnostics by enabling rapid, accurate point-of-care testing with minimal sample volumes, outperforming traditional central lab methods in speed and portability.
Sample-to-answer systems
Sample-to-answer systems in point-of-care devices deliver rapid, accurate diagnostic results by integrating sample preparation, amplification, and detection within a compact platform, significantly reducing turnaround time compared to central lab diagnostics.
Turnaround time
Point-of-care devices deliver diagnostic results within minutes at the patient's location, significantly reducing turnaround time compared to central lab diagnostics that typically require hours to process and report results due to sample transportation and batching.
Analytical sensitivity
Point-of-care devices typically exhibit lower analytical sensitivity compared to central lab diagnostics, impacting their ability to detect low-concentration biomarkers accurately.
Quality control protocols
Point-of-care devices implement streamlined quality control protocols optimized for rapid, on-site testing, whereas central lab diagnostics maintain comprehensive, multi-step quality assurance processes to ensure higher analytical precision and regulatory compliance.
Decentralized testing
Decentralized testing with point-of-care devices enables rapid, onsite diagnostics that improve patient outcomes by reducing turnaround time compared to traditional central lab diagnostics.
Reference laboratory validation
Reference laboratory validation ensures the accuracy and reliability of point-of-care devices by systematically comparing their diagnostic performance against established central lab standards.
Multiplexed biomarker detection
Multiplexed biomarker detection in point-of-care devices enables rapid, simultaneous analysis of multiple targets with enhanced clinical efficiency compared to centralized lab diagnostics.
point-of-care devices vs central lab diagnostics Infographic
 njnir.com
njnir.com