PFAS and microplastics pose distinct environmental challenges due to their persistence and widespread contamination. PFAS are synthetic chemicals that bioaccumulate in water sources and living organisms, creating long-term toxicity risks. Microplastics, fragmented from larger plastic debris, infiltrate aquatic ecosystems, disrupting food chains and causing physical harm to marine life.
Table of Comparison
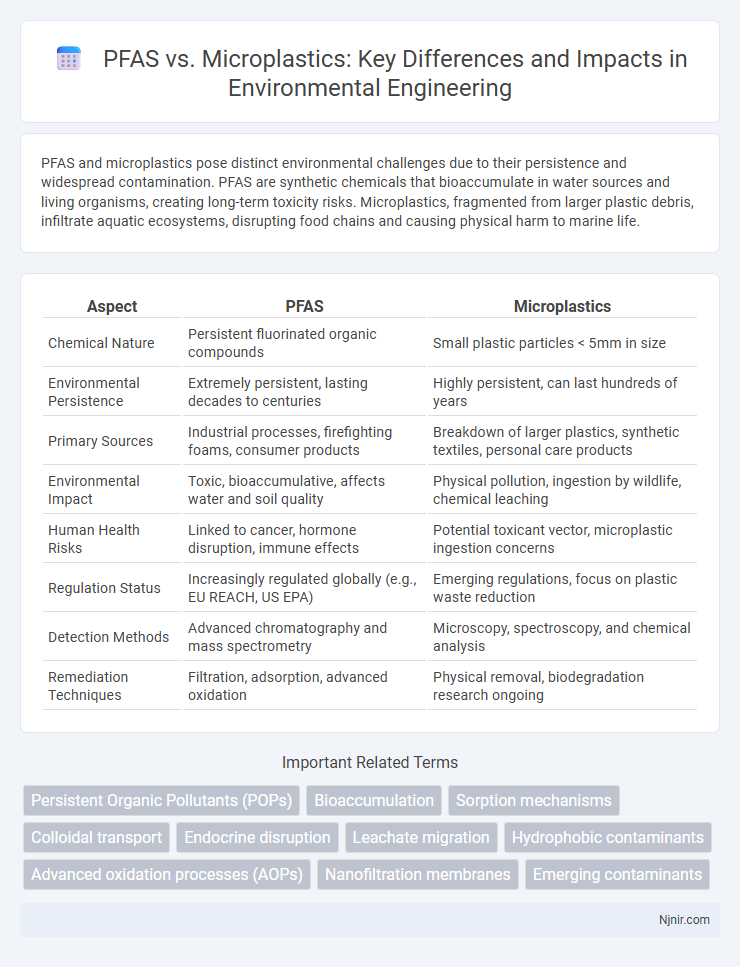
| Aspect | PFAS | Microplastics |
|---|---|---|
| Chemical Nature | Persistent fluorinated organic compounds | Small plastic particles < 5mm in size |
| Environmental Persistence | Extremely persistent, lasting decades to centuries | Highly persistent, can last hundreds of years |
| Primary Sources | Industrial processes, firefighting foams, consumer products | Breakdown of larger plastics, synthetic textiles, personal care products |
| Environmental Impact | Toxic, bioaccumulative, affects water and soil quality | Physical pollution, ingestion by wildlife, chemical leaching |
| Human Health Risks | Linked to cancer, hormone disruption, immune effects | Potential toxicant vector, microplastic ingestion concerns |
| Regulation Status | Increasingly regulated globally (e.g., EU REACH, US EPA) | Emerging regulations, focus on plastic waste reduction |
| Detection Methods | Advanced chromatography and mass spectrometry | Microscopy, spectroscopy, and chemical analysis |
| Remediation Techniques | Filtration, adsorption, advanced oxidation | Physical removal, biodegradation research ongoing |
Introduction to PFAS and Microplastics
Per- and polyfluoroalkyl substances (PFAS) are a group of synthetic chemicals widely used for their resistance to heat, water, and oil, raising concerns due to their persistence and bioaccumulation in the environment. Microplastics, tiny plastic particles less than 5 millimeters in diameter, originate from the breakdown of larger plastic debris and are pervasive contaminants in marine and terrestrial ecosystems. Both PFAS and microplastics pose significant environmental and health risks due to their persistence, potential toxicity, and widespread presence in food, water, and air.
Sources and Pathways in the Environment
PFAS primarily enter the environment through industrial discharges, firefighting foams, and wastewater treatment plant effluents, contaminating soil and water sources. Microplastics originate from degraded plastic waste, synthetic fibers, and personal care products, dispersing through atmospheric deposition, river runoff, and ocean currents. Both contaminants persist in various environmental matrices, bioaccumulating in aquatic and terrestrial food webs due to their widespread sources and complex pathways.
Chemical Structures and Properties
PFAS (per- and polyfluoroalkyl substances) are characterized by strong carbon-fluorine bonds that create highly stable, hydrophobic, and lipophobic molecules resistant to heat and chemical degradation. Microplastics consist of various polymer fragments, such as polyethylene, polypropylene, and polystyrene, with diverse chemical structures that influence their buoyancy, degradation rates, and interaction with environmental contaminants. The chemical inertness of PFAS contrasts with the physical and chemical diversity of microplastics, affecting their environmental persistence and pathways of bioaccumulation.
Environmental Fate and Transport
PFAS (per- and polyfluoroalkyl substances) exhibit persistence in environmental media due to their strong carbon-fluorine bonds, leading to widespread detection in water, soil, and biota through long-range atmospheric transport and groundwater contamination. Microplastics, composed of diverse polymer fragments, undergo physical fragmentation, settling, or resuspension in aquatic and terrestrial systems, often serving as vectors for chemical pollutants and microorganisms. Both contaminants demonstrate complex environmental transport pathways influenced by particle size, chemical properties, and interactions with biotic and abiotic factors, contributing to their global distribution and ecological risks.
Detection and Measurement Techniques
Detection and measurement techniques for PFAS primarily involve high-resolution mass spectrometry (HRMS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to identify and quantify trace concentrations in environmental and biological samples. Microplastics detection relies on Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy for polymer identification, alongside microscopy methods like scanning electron microscopy (SEM) for morphological analysis. Emerging methods such as pyrolysis-GC-MS offer precise quantification of both PFAS and microplastics, enhancing sensitivity and specificity in complex matrices.
Health and Ecological Impacts
PFAS (per- and polyfluoroalkyl substances) persist in the environment and bioaccumulate, causing immune system disruption, hormone interference, and increased cancer risk in humans. Microplastics infiltrate aquatic ecosystems, damaging marine life through ingestion and chemical toxicity, leading to bioaccumulation and food web contamination. Both PFAS and microplastics contribute to long-term ecological imbalance and pose significant health risks due to their persistence and bioaccumulative properties.
Regulatory Frameworks and Guidelines
Regulatory frameworks for PFAS (per- and polyfluoroalkyl substances) focus primarily on strict limits for chemical concentrations in water, soil, and consumer products due to their persistence and bioaccumulation, with agencies like the U.S. EPA setting health advisory levels and state-specific restrictions. Microplastics regulations are emerging globally, emphasizing monitoring protocols and restrictions on microbeads in cosmetics and wastewater discharge, as seen in EU directives and national bans targeting plastic pollution management. Both PFAS and microplastics face evolving guidelines that increasingly prioritize environmental risk assessment, remediation strategies, and international cooperation to address their widespread contamination.
Treatment and Remediation Technologies
Advanced treatment technologies for PFAS prioritize activated carbon adsorption, ion exchange resins, and high-pressure membrane filtration to effectively remove persistent fluorinated compounds from water sources. Microplastic remediation often involves enhanced filtration methods, including microfiltration and ultrafiltration membranes, combined with coagulation-flocculation processes to capture diverse polymer particles. Emerging approaches such as advanced oxidation processes (AOPs) and biodegradation show promise in degrading PFAS molecules and breaking down microplastics, respectively, addressing critical environmental contamination challenges.
Challenges in Management and Mitigation
PFAS and microplastics pose significant challenges in environmental management due to their persistent nature and widespread distribution. PFAS chemicals resist degradation, complicating removal from water sources and requiring advanced treatment technologies like activated carbon filtration or ion exchange resins. Microplastics evade conventional filtration systems, accumulate in ecosystems, and demand innovative solutions such as microbial biodegradation or enhanced capture methods for effective mitigation.
Future Directions and Research Priorities
Future research on PFAS and microplastics should prioritize the development of advanced detection technologies to improve identification of these contaminants at trace levels in diverse environmental matrices. Investigating the synergistic effects and combined toxicity of PFAS and microplastics on human health and ecosystems is critical for comprehensive risk assessment. Emphasis on sustainable remediation techniques and regulatory frameworks will support mitigation efforts and promote long-term environmental protection.
Persistent Organic Pollutants (POPs)
PFAS and microplastics are both persistent organic pollutants (POPs) with PFAS comprising synthetic fluorinated chemicals known for bioaccumulation and toxicity, while microplastics act as physical vectors for POPs, enabling their transport and environmental persistence.
Bioaccumulation
PFAS exhibit higher bioaccumulation potential in aquatic organisms compared to microplastics, leading to more persistent and toxic effects in food chains.
Sorption mechanisms
PFAS exhibit high sorption to organic matter and hydrophobic surfaces due to their fluorinated chains, while microplastics primarily sorb contaminants through surface adsorption influenced by polymer type, aging, and surface area.
Colloidal transport
Colloidal transport enables PFAS to persistently migrate through soil and water matrices more effectively than microplastics, influencing their environmental distribution and remediation challenges.
Endocrine disruption
PFAS and microplastics both pose significant endocrine disruption risks, with PFAS interfering primarily through hormone receptor binding and microplastics acting as carriers for hormone-disrupting chemicals.
Leachate migration
Leachate migration of PFAS poses greater environmental persistence and toxicity risks compared to microplastics due to PFAS's higher solubility and mobility in water systems.
Hydrophobic contaminants
PFAS and microplastics are hydrophobic contaminants that persist in the environment, with PFAS exhibiting strong chemical stability and microplastics serving as vectors for adsorbing and transporting other hydrophobic pollutants in aquatic ecosystems.
Advanced oxidation processes (AOPs)
Advanced oxidation processes (AOPs) effectively degrade PFAS and microplastics by generating reactive radicals that break down persistent chemical bonds and polymer structures.
Nanofiltration membranes
Nanofiltration membranes effectively remove PFAS and microplastics from water by targeting nanoparticles and organic contaminants, offering a specialized treatment solution for emerging pollutants.
Emerging contaminants
PFAS and microplastics are emerging contaminants with persistent environmental presence, posing significant risks to human health and ecosystems due to their widespread distribution and resistance to degradation.
PFAS vs microplastics Infographic
 njnir.com
njnir.com