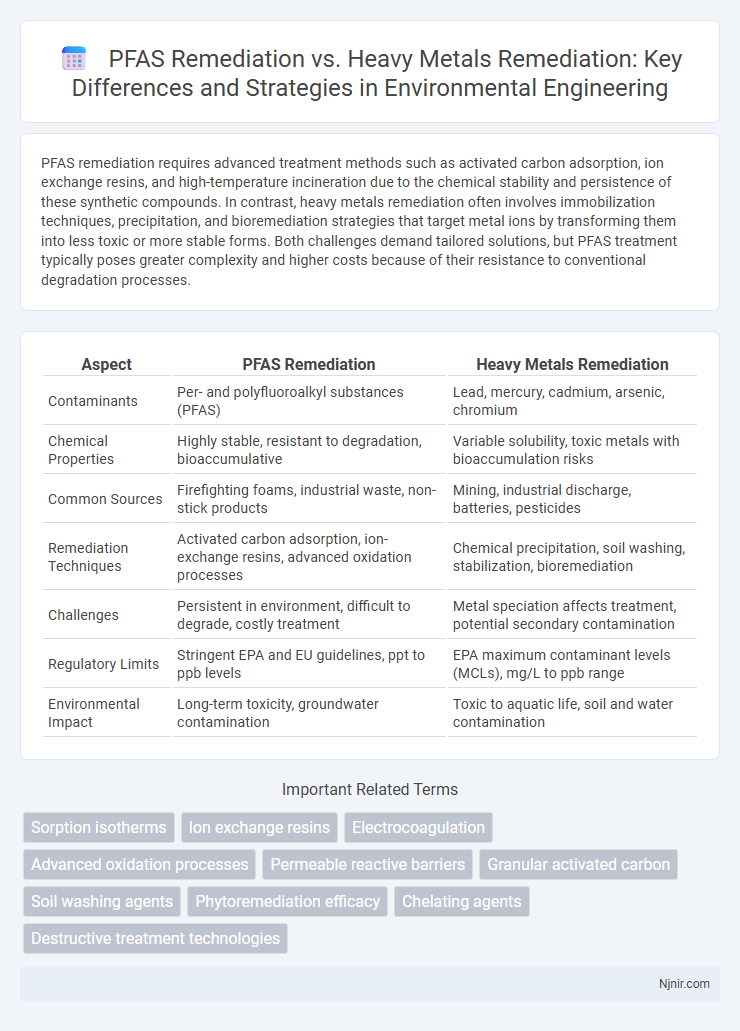
PFAS remediation requires advanced treatment methods such as activated carbon adsorption, ion exchange resins, and high-temperature incineration due to the chemical stability and persistence of these synthetic compounds. In contrast, heavy metals remediation often involves immobilization techniques, precipitation, and bioremediation strategies that target metal ions by transforming them into less toxic or more stable forms. Both challenges demand tailored solutions, but PFAS treatment typically poses greater complexity and higher costs because of their resistance to conventional degradation processes.
Table of Comparison
| Aspect | PFAS Remediation | Heavy Metals Remediation |
|---|---|---|
| Contaminants | Per- and polyfluoroalkyl substances (PFAS) | Lead, mercury, cadmium, arsenic, chromium |
| Chemical Properties | Highly stable, resistant to degradation, bioaccumulative | Variable solubility, toxic metals with bioaccumulation risks |
| Common Sources | Firefighting foams, industrial waste, non-stick products | Mining, industrial discharge, batteries, pesticides |
| Remediation Techniques | Activated carbon adsorption, ion-exchange resins, advanced oxidation processes | Chemical precipitation, soil washing, stabilization, bioremediation |
| Challenges | Persistent in environment, difficult to degrade, costly treatment | Metal speciation affects treatment, potential secondary contamination |
| Regulatory Limits | Stringent EPA and EU guidelines, ppt to ppb levels | EPA maximum contaminant levels (MCLs), mg/L to ppb range |
| Environmental Impact | Long-term toxicity, groundwater contamination | Toxic to aquatic life, soil and water contamination |
Understanding PFAS and Heavy Metal Contamination
PFAS remediation involves targeting persistent, synthetic chemicals resistant to degradation and commonly found in industrial sites and firefighting foam locations, whereas heavy metals remediation focuses on removing toxic elements like lead, mercury, and arsenic from contaminated soil and water. PFAS contamination presents unique challenges due to its chemical stability and widespread presence in water supplies, requiring advanced filtration and chemical treatment methods. Heavy metals contamination is often addressed using physical or chemical immobilization techniques, soil washing, or bioremediation to reduce toxicity and prevent bioaccumulation.
Sources and Environmental Impact of PFAS vs Heavy Metals
PFAS contamination primarily originates from industrial applications, firefighting foams, and consumer products, persisting in water bodies and soil due to their strong carbon-fluorine bonds, leading to bioaccumulation and adverse health effects. Heavy metals such as lead, mercury, and cadmium stem from mining, industrial waste, and agricultural runoff, causing toxic accumulation in ecosystems and posing severe risks to flora, fauna, and human health. The environmental impact of PFAS is characterized by their widespread persistence and resistance to degradation, while heavy metals cause long-term soil and water toxicity through bio-magnification and disruption of biological processes.
Chemical Properties Affecting Remediation Processes
PFAS remediation challenges arise from their strong carbon-fluorine bonds, chemical stability, and hydrophobic and lipophobic characteristics, which resist breakdown in conventional treatment methods. Heavy metals remediation focuses on the metal ions' variable oxidation states, solubility, and affinity for sorbents, enabling processes like precipitation, adsorption, and ion exchange. The contrasting chemical properties demand tailored approaches: advanced oxidation for PFAS to disrupt C-F bonds, versus physicochemical methods exploiting metal speciation for heavy metals removal.
Regulatory Frameworks for PFAS and Heavy Metals
Regulatory frameworks for PFAS (per- and polyfluoroalkyl substances) remediation are increasingly stringent, driven by EPA's recent health advisories and evolving state-level limits emphasizing zero or near-zero detection thresholds. Heavy metals remediation is governed by established regulations such as the Clean Water Act and Toxic Substances Control Act, with well-defined maximum contaminant levels (MCLs) for metals like lead, mercury, and arsenic. PFAS regulation remains challenging due to persistence and bioaccumulation, prompting new policies focusing on source control, treatment technologies, and long-term monitoring compared to the more mature regulatory processes for heavy metals.
Overview of Conventional Remediation Techniques
Conventional remediation techniques for PFAS focus primarily on adsorption using activated carbon, ion exchange resins, and membrane filtration, which effectively capture these persistent per- and polyfluoroalkyl substances from contaminated water. Heavy metals remediation commonly employs chemical precipitation, ion exchange, and soil washing to immobilize or remove metals like lead, cadmium, and arsenic from soil and water. While both contaminant types benefit from sorption methods, PFAS require specialized treatment due to their chemical stability and resistance to biodegradation compared to metals, which can often be stabilized or chemically transformed into less toxic forms.
Emerging Technologies in PFAS Remediation
Emerging technologies in PFAS remediation include advanced oxidation processes, electrochemical treatment, and novel adsorbents like functionalized carbon nanotubes, which target the chemically stable PFAS molecules more effectively than traditional methods used in heavy metals remediation. Unlike heavy metals remediation that often relies on precipitation, ion exchange, or bioremediation, PFAS remediation challenges stem from the strong C-F bonds, necessitating innovative approaches such as photocatalysis and plasma treatment. Ongoing research prioritizes scalable, cost-effective solutions to degrade or remove per- and polyfluoroalkyl substances from contaminated water and soil, addressing their persistence and health risks.
Innovative Solutions for Heavy Metals Removal
Innovative solutions for heavy metals removal include advanced materials such as biochar, graphene oxide, and metal-organic frameworks (MOFs) that exhibit high adsorption capacities and selectivity for toxic metals like lead, mercury, and cadmium. Electrochemical methods, including electrodialysis and electrocoagulation, offer energy-efficient and scalable approaches to precipitate and recover heavy metals from wastewater. These cutting-edge technologies contrast with PFAS remediation techniques, which often rely on destructive methods like advanced oxidation or adsorption on activated carbon, highlighting the tailored strategies required for differing pollutant chemistries.
Comparative Analysis: Effectiveness and Challenges
PFAS remediation presents unique challenges due to the chemical stability and persistence of per- and polyfluoroalkyl substances, requiring advanced methods like activated carbon adsorption, ion exchange resins, and high-temperature incineration, while heavy metals remediation often relies on established techniques such as chemical precipitation, ion exchange, and bioremediation targeting metal ions like lead, mercury, and cadmium. Effectiveness in PFAS removal depends heavily on compound-specific properties and treatment technology, whereas heavy metals remediation benefits from well-documented removal efficiencies but faces challenges such as secondary waste generation and metal bioaccumulation risks. Both remediation approaches demand site-specific assessment, balancing treatment costs, environmental impact, and regulatory standards to optimize pollutant reduction and ensure long-term groundwater and soil safety.
Environmental and Public Health Risks Post-Remediation
PFAS remediation often involves challenges due to the chemical's persistence and bioaccumulation potential, posing ongoing risks of groundwater contamination and adverse health effects such as immune system disruption and cancer even after treatment. Heavy metals remediation prioritizes removing toxic elements like lead and mercury to prevent neurotoxicity, kidney damage, and developmental issues, but residual soil contamination can still pose chronic exposure risks. Effective post-remediation monitoring is crucial in both cases to ensure contaminant levels remain below EPA safety thresholds, minimizing long-term environmental and public health hazards.
Future Directions in PFAS and Heavy Metal Remediation
Future directions in PFAS remediation emphasize advanced oxidation processes, ion exchange resins, and sustainable adsorbents targeting persistent per- and polyfluoroalkyl substances in contaminated sites. Heavy metals remediation is increasingly exploring nanotechnology-based materials and biochar to immobilize or extract toxic metals like lead, cadmium, and arsenic from soil and water. Integrating real-time monitoring with machine learning models enhances site-specific treatment strategies, optimizing the efficiency and cost-effectiveness of both PFAS and heavy metal remediation technologies.
Sorption isotherms
Sorption isotherms for PFAS remediation typically exhibit nonlinear Freundlich behavior due to their organic fluorinated structure, whereas heavy metals remediation often follows linear or Langmuir isotherms reflecting metal ion adsorption on mineral surfaces.
Ion exchange resins
Ion exchange resins efficiently remove heavy metals through strong ionic binding but face challenges in PFAS remediation due to the complex fluorinated structures requiring specialized resin formulations for effective adsorption.
Electrocoagulation
Electrocoagulation efficiently removes PFAS and heavy metals by generating coagulants in situ, with PFAS remediation requiring optimized electrode materials and operating parameters due to their persistent chemical structure compared to heavy metals.
Advanced oxidation processes
Advanced oxidation processes (AOPs) effectively degrade PFAS compounds by generating reactive radicals, while heavy metals remediation relies on AOPs primarily for transforming associated organic contaminants rather than direct metal removal.
Permeable reactive barriers
Permeable reactive barriers effectively target PFAS contamination by employing specialized reactive media for long-term degradation, whereas heavy metals remediation relies on sorptive or precipitative materials within the barriers to immobilize and prevent metal migration.
Granular activated carbon
Granular activated carbon is highly effective for PFAS remediation due to its strong adsorption properties, whereas heavy metals remediation often requires complementary treatments like ion exchange or chemical precipitation for optimal removal.
Soil washing agents
Soil washing agents designed for PFAS remediation typically target fluorinated compounds using advanced surfactants and co-solvents, whereas heavy metals remediation relies on chelating agents and acid-based solutions to effectively remove metallic contaminants from soil.
Phytoremediation efficacy
Phytoremediation demonstrates higher efficacy in heavy metals remediation due to plant uptake and accumulation mechanisms, whereas PFAS remediation is limited by the persistent and chemically stable nature of PFAS compounds, reducing plant absorption and degradation.
Chelating agents
Chelating agents are extensively used in heavy metals remediation to bind and immobilize toxic metals, whereas PFAS remediation relies less on chelation and more on advanced oxidation and adsorption techniques due to PFAS's stable fluorinated structure.
Destructive treatment technologies
Destructive treatment technologies for PFAS remediation include advanced oxidation processes and plasma treatment that break chemical bonds, whereas heavy metals remediation often employs stabilization and chemical reduction to transform or immobilize contaminants.
PFAS remediation vs heavy metals remediation Infographic
 njnir.com
njnir.com