PFAS remediation presents unique challenges due to the chemical stability and persistence of per- and polyfluoroalkyl substances, requiring advanced oxidation and adsorption techniques for effective removal. In contrast, heavy metal remediation often relies on chemical precipitation, ion exchange, and bioremediation methods to immobilize or extract toxic metals from contaminated sites. Tailoring remediation strategies to specific contaminants enhances treatment efficiency and minimizes environmental impact.
Table of Comparison
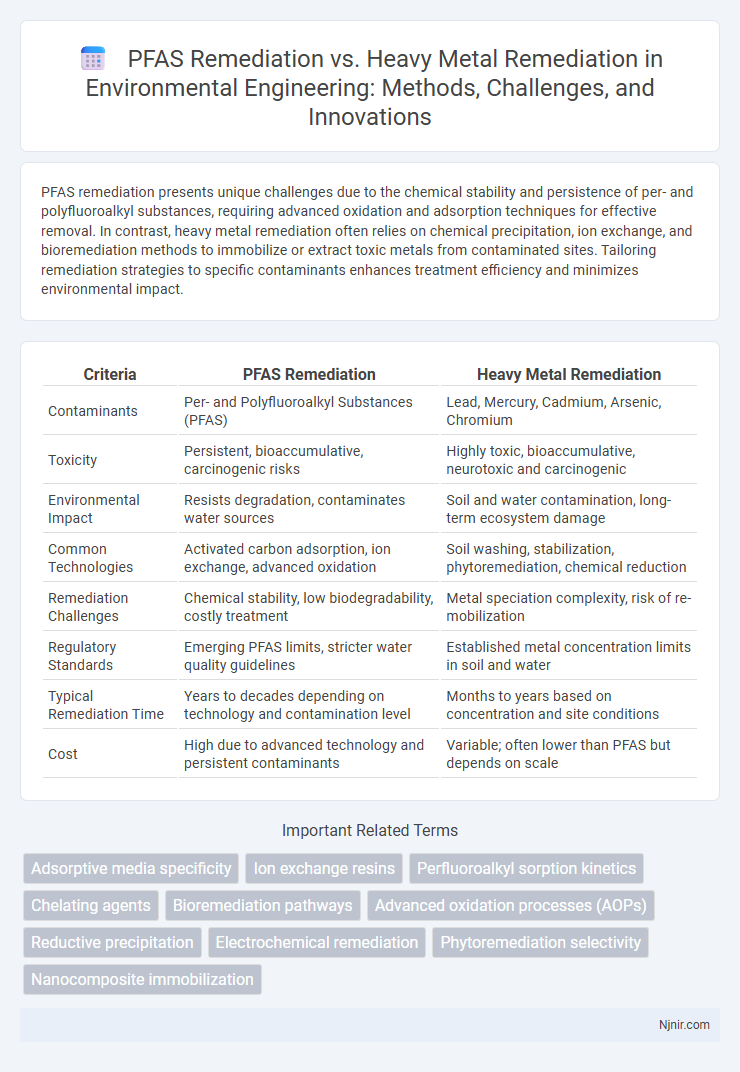
| Criteria | PFAS Remediation | Heavy Metal Remediation |
|---|---|---|
| Contaminants | Per- and Polyfluoroalkyl Substances (PFAS) | Lead, Mercury, Cadmium, Arsenic, Chromium |
| Toxicity | Persistent, bioaccumulative, carcinogenic risks | Highly toxic, bioaccumulative, neurotoxic and carcinogenic |
| Environmental Impact | Resists degradation, contaminates water sources | Soil and water contamination, long-term ecosystem damage |
| Common Technologies | Activated carbon adsorption, ion exchange, advanced oxidation | Soil washing, stabilization, phytoremediation, chemical reduction |
| Remediation Challenges | Chemical stability, low biodegradability, costly treatment | Metal speciation complexity, risk of re-mobilization |
| Regulatory Standards | Emerging PFAS limits, stricter water quality guidelines | Established metal concentration limits in soil and water |
| Typical Remediation Time | Years to decades depending on technology and contamination level | Months to years based on concentration and site conditions |
| Cost | High due to advanced technology and persistent contaminants | Variable; often lower than PFAS but depends on scale |
Introduction to PFAS and Heavy Metal Contamination
PFAS (per- and polyfluoroalkyl substances) contamination involves persistent synthetic chemicals widely found in industrial sites, posing long-term risks to water sources and human health due to their resistance to degradation. Heavy metal contamination typically results from mining, industrial discharge, and improper waste disposal, introducing toxic metals such as lead, arsenic, and mercury into soils and groundwater. Both PFAS and heavy metals require specialized remediation technologies tailored to their chemical persistence and environmental toxicity profiles.
Sources and Environmental Impact of PFAS vs Heavy Metals
PFAS contamination primarily originates from industrial sites, firefighting foams, and consumer products, persisting in water, soil, and biota due to their chemical stability and bioaccumulative nature. Heavy metals such as lead, mercury, and cadmium enter the environment through mining, industrial emissions, and improper waste disposal, causing toxicity in ecosystems and human health through bioaccumulation and soil contamination. The persistent, widespread nature of PFAS and their resistance to conventional remediation contrasts with the localized but highly toxic impact of heavy metals, necessitating distinct environmental management strategies.
Chemical Properties Influencing Remediation Approaches
PFAS remediation requires addressing the strong carbon-fluorine bonds and hydrophobic-lipophobic nature of these persistent organic pollutants, which limits degradation by conventional methods. Heavy metal remediation focuses on the metals' ionic properties, oxidation states, and solubility, enabling approaches like adsorption, precipitation, and ion exchange. The distinct chemical stability and polarity differences between PFAS and heavy metals necessitate tailored techniques, such as advanced oxidation for PFAS and chemical reduction or chelation for metals.
Regulatory Standards for PFAS and Heavy Metals
Regulatory standards for PFAS remediation are becoming increasingly stringent worldwide due to PFAS's persistence and bioaccumulative toxicity, with agencies like the U.S. EPA setting advisory limits as low as 70 parts per trillion in drinking water. Heavy metal remediation standards vary by metal type, with toxins such as lead, mercury, and arsenic regulated under well-established frameworks like the Safe Drinking Water Act, often permitting concentrations in the parts per billion range. Compliance with these regulations drives tailored remediation technologies, as PFAS requires specialized advanced treatment methods compared to more conventional heavy metal removal processes.
Conventional Remediation Techniques for Heavy Metals
Conventional remediation techniques for heavy metals primarily involve soil excavation, stabilization, and chemical precipitation to reduce toxicity and mobility. These methods effectively immobilize contaminants such as lead, cadmium, and mercury, minimizing environmental and health risks. In contrast, PFAS remediation requires advanced technologies like adsorption using activated carbon or ion exchange resins, due to the persistent and resistant nature of per- and polyfluoroalkyl substances.
Emerging Remediation Technologies for PFAS
Emerging remediation technologies for PFAS focus on advanced oxidation processes, electrochemical treatment, and novel adsorbents like biochar and ion-exchange resins to effectively target persistent per- and polyfluoroalkyl substances. These methods aim to overcome the chemical stability and resistance of PFAS, which differ significantly from heavy metal remediation that primarily relies on precipitation, stabilization, and soil washing techniques. Innovations such as plasma treatment and photocatalysis show promise in breaking the strong carbon-fluorine bonds unique to PFAS contamination, highlighting the distinct approaches required compared to traditional heavy metal cleanup strategies.
Challenges in PFAS Remediation Compared to Heavy Metals
PFAS remediation faces unique challenges due to the chemical stability and persistence of per- and polyfluoroalkyl substances, which resist conventional treatment methods like adsorption or chemical precipitation effective in heavy metal removal. Unlike heavy metals that can often be immobilized or extracted through established remediation techniques such as ion exchange or precipitation, PFAS compounds require advanced technologies like high-pressure membranes, advanced oxidation processes, or specialized sorbents to achieve significant degradation or removal. The recalcitrant nature and low biodegradability of PFAS increase the complexity, cost, and time needed for remediation compared to heavy metals, posing significant environmental management obstacles.
Cost Analysis: PFAS Remediation versus Heavy Metal Remediation
PFAS remediation generally incurs higher costs than heavy metal remediation due to the persistent and complex nature of PFAS chemicals, requiring advanced treatment technologies such as granular activated carbon, ion exchange, or high-pressure membranes. Heavy metal remediation often utilizes more established and cost-effective methods like chemical precipitation, soil washing, and stabilization, which typically demand less energy and materials. Budget allocation for PFAS projects must consider longer treatment times and disposal expenses, while heavy metal cleanup costs frequently center around site characterization and contaminant extraction efficiencies.
Long-Term Effectiveness and Sustainability of Remediation Methods
PFAS remediation methods like activated carbon adsorption and advanced oxidation demonstrate varied long-term effectiveness due to the persistent and bioaccumulative nature of PFAS compounds, often requiring continual monitoring and repeated treatment cycles. Heavy metal remediation techniques such as soil washing, stabilization, and phytoremediation typically offer more sustained outcomes by permanently immobilizing or extracting contaminants, though effectiveness depends on soil chemistry and metal speciation. Sustainable remediation prioritizes minimizing secondary waste, energy consumption, and ecological disruption, with integrated approaches increasingly favored to address the complex behavior and longevity of both PFAS and heavy metals in the environment.
Future Perspectives in Contaminant Remediation Strategies
Emerging contaminant remediation strategies increasingly prioritize advanced technologies such as electrochemical oxidation and photocatalysis for PFAS removal, addressing their persistent and complex molecular structures. Heavy metal remediation emphasizes bioremediation and nanomaterials that enhance metal adsorption and transformation, promoting sustainable and cost-effective solutions. Future perspectives highlight integrating hybrid systems combining physical, chemical, and biological methods to achieve higher efficiency and target multiple contaminants simultaneously.
Adsorptive media specificity
Adsorptive media for PFAS remediation typically require fluoropolymer-based materials with high affinity for perfluorinated compounds, whereas heavy metal remediation relies on media such as activated carbon or ion-exchange resins specifically tailored for metal ion adsorption.
Ion exchange resins
Ion exchange resins effectively remove PFAS by targeting their ionic charges, whereas heavy metal remediation with these resins relies on selective adsorption of metal ions based on resin functional groups.
Perfluoroalkyl sorption kinetics
Perfluoroalkyl sorption kinetics exhibit slower adsorption rates and lower equilibrium capacities in PFAS remediation compared to the typically faster and higher-capacity sorption processes observed in heavy metal remediation.
Chelating agents
Chelating agents effectively bind heavy metals for remediation by forming stable complexes, whereas PFAS remediation requires specialized adsorbents or advanced oxidation processes due to PFAS's persistent fluorinated carbon chains resistant to chelation.
Bioremediation pathways
Bioremediation pathways for PFAS remediation primarily involve specialized microbial enzymes that degrade perfluorinated compounds, whereas heavy metal bioremediation relies on microbial biosorption, bioaccumulation, and enzymatic transformation to immobilize or detoxify metals.
Advanced oxidation processes (AOPs)
Advanced oxidation processes (AOPs) effectively degrade PFAS through radical generation but require tailored conditions for heavy metal remediation, which often involves additional treatment steps like precipitation or adsorption.
Reductive precipitation
Reductive precipitation effectively immobilizes heavy metals by converting them into insoluble forms, while its application to PFAS remediation remains challenging due to the chemical stability of PFAS compounds.
Electrochemical remediation
Electrochemical remediation effectively targets both PFAS and heavy metals by using controlled electric currents to degrade complex PFAS molecules and mobilize heavy metal ions for extraction from contaminated sites.
Phytoremediation selectivity
Phytoremediation selectively targets PFAS by utilizing specific plant species capable of accumulating and degrading per- and polyfluoroalkyl substances, whereas heavy metal remediation often relies on hyperaccumulator plants that absorb and immobilize metals without degradation.
Nanocomposite immobilization
Nanocomposite immobilization enhances PFAS remediation by providing superior adsorption and degradation capabilities compared to heavy metal remediation, which primarily relies on nanoparticle adsorption and precipitation mechanisms.
PFAS remediation vs heavy metal remediation Infographic
 njnir.com
njnir.com