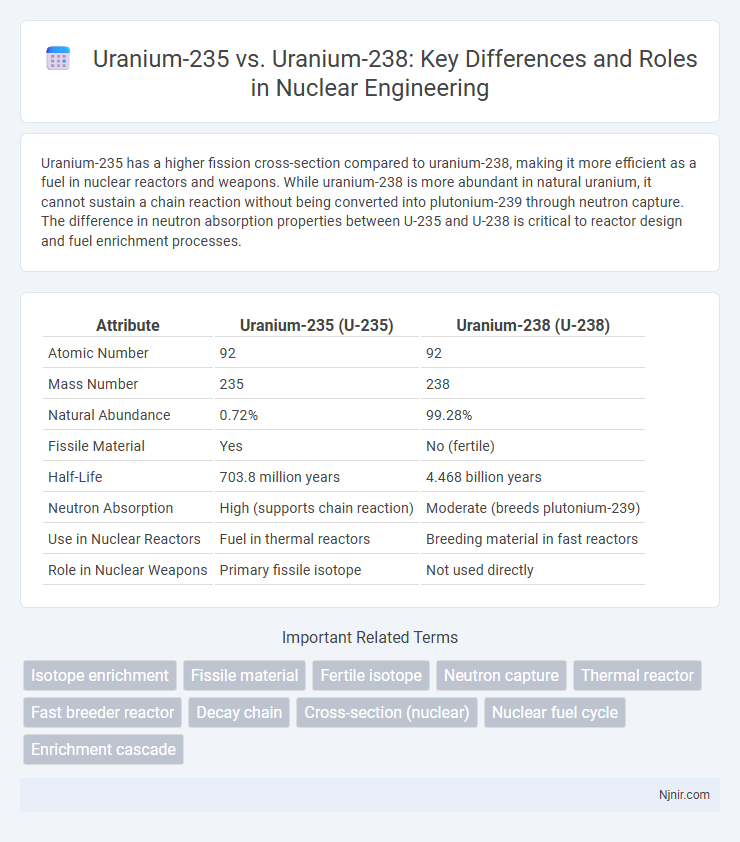
Uranium-235 has a higher fission cross-section compared to uranium-238, making it more efficient as a fuel in nuclear reactors and weapons. While uranium-238 is more abundant in natural uranium, it cannot sustain a chain reaction without being converted into plutonium-239 through neutron capture. The difference in neutron absorption properties between U-235 and U-238 is critical to reactor design and fuel enrichment processes.
Table of Comparison
| Attribute | Uranium-235 (U-235) | Uranium-238 (U-238) |
|---|---|---|
| Atomic Number | 92 | 92 |
| Mass Number | 235 | 238 |
| Natural Abundance | 0.72% | 99.28% |
| Fissile Material | Yes | No (fertile) |
| Half-Life | 703.8 million years | 4.468 billion years |
| Neutron Absorption | High (supports chain reaction) | Moderate (breeds plutonium-239) |
| Use in Nuclear Reactors | Fuel in thermal reactors | Breeding material in fast reactors |
| Role in Nuclear Weapons | Primary fissile isotope | Not used directly |
Isotopic Composition: Uranium-235 vs Uranium-238
Uranium-235 constitutes about 0.72% of natural uranium, while uranium-238 accounts for roughly 99.27%, defining their isotopic composition. Uranium-235 is fissile and crucial for nuclear reactors and weapons, whereas uranium-238 is fertile, capable of converting into plutonium-239 through neutron absorption. The significant disparity in abundance impacts uranium enrichment processes vital for fuel preparation and nuclear technology applications.
Nuclear Properties and Reactivity
Uranium-235 exhibits a higher reactivity due to its fissile nature, enabling it to sustain a nuclear chain reaction with thermal neutrons, unlike uranium-238, which is fertile and primarily undergoes neutron capture to form plutonium-239. The nuclear properties of uranium-235 include a half-life of approximately 703.8 million years and a significant cross-section for fission, making it critical for nuclear reactors and weapons. Uranium-238's longer half-life of about 4.468 billion years and lower fission cross-section contribute to its role as a nuclear fuel breeder rather than a direct fissile material.
Abundance and Natural Occurrence
Uranium-238 constitutes about 99.3% of natural uranium, making it the most abundant isotope found in nature, while uranium-235 accounts for only approximately 0.7%. The higher natural abundance of uranium-238 contributes to its dominance in uranium deposits, though uranium-235 is critical for nuclear fission due to its fissile properties. This disparity in abundance impacts uranium enrichment processes required for nuclear fuel and weapons production.
Role in Nuclear Power Generation
Uranium-235 plays a crucial role in nuclear power generation as it is the primary fissile isotope used to sustain chain reactions in nuclear reactors, releasing significant energy. Uranium-238, while more abundant, is not fissile but fertile, as it can absorb neutrons and transmute into plutonium-239, which is also fissile and contributes to energy production. The balance between U-235's direct fission and U-238's breeding capabilities determines reactor fuel efficiency and longevity.
Nuclear Fission: Comparison of Mechanisms
Uranium-235 undergoes nuclear fission more readily than uranium-238 due to its ability to sustain a chain reaction with thermal neutrons, making it a primary fuel in nuclear reactors and weapons. Uranium-238 primarily captures fast neutrons and undergoes fission less efficiently, serving mostly as a fertile material that can absorb neutrons and transmute into plutonium-239. The distinct fission mechanisms result from their nuclear structure differences, with Uranium-235 having a lower neutron absorption cross-section for slow neutrons compared to Uranium-238.
Uranium Enrichment Processes
Uranium enrichment processes increase the concentration of uranium-235 isotopes from its natural level of 0.7% to higher levels suitable for nuclear reactors or weapons. Gas centrifuge and gaseous diffusion techniques separate uranium-235 from uranium-238 based on their slight mass differences, enhancing fissile material content. Enriched uranium typically contains 3-5% uranium-235 for reactor fuel, while weapons-grade uranium exceeds 90% uranium-235.
Applications in Nuclear Weapons and Fuel
Uranium-235, due to its fissile properties, is a critical component in nuclear weapons and is widely used as fuel in nuclear reactors to sustain chain reactions. Uranium-238, while not fissile, serves as a fertile material that transforms into plutonium-239, which is also weaponizable and utilized as reactor fuel. The enrichment process increases the concentration of U-235 in uranium to levels suitable for weapons or reactor applications, reflecting their distinct but complementary roles in nuclear technology.
Radioactive Decay and Half-Life
Uranium-235 undergoes alpha decay with a half-life of approximately 703.8 million years, making it significantly less stable but more fissile than Uranium-238, which has a half-life of about 4.468 billion years. The longer half-life of Uranium-238 results in a slower decay rate, contributing to its abundance in natural uranium. Differences in radioactive decay rates influence their applications in nuclear reactors; Uranium-235's higher reactivity enables efficient nuclear fission, whereas Uranium-238 primarily serves as fertile material for breeding plutonium-239.
Challenges in Handling and Storage
Uranium-235 presents greater challenges in handling and storage due to its higher radioactivity and fissile properties, requiring stringent shielding and criticality safety measures to prevent accidental chain reactions. Uranium-238, though less radioactive and not directly fissile, demands careful containment to avoid environmental contamination and long-term radiological risks from its decay products. Both isotopes necessitate secure, stable storage facilities to mitigate risks of radiation exposure, theft, and proliferation concerns.
Future Prospects and Innovations
Uranium-235's high fissile properties make it essential for next-generation nuclear reactors aimed at safer, more efficient energy production, while Uranium-238's abundance supports advanced breeder reactors that generate additional fissile material. Innovations in enrichment technology and fast neutron reactors are enhancing the utilization efficiency of both isotopes, reducing nuclear waste and extending fuel resources. Research into thorium fuel cycles and hybrid reactors also leverages Uranium-238's neutron absorption capabilities, promising sustainable nuclear energy solutions for the future.
Isotope enrichment
Uranium-235 enrichment increases its concentration from 0.72% to 3-5% for nuclear fuel, whereas uranium-238 remains the predominant isotope at about 95%, making isotope separation essential for reactor-grade uranium.
Fissile material
Uranium-235 is a fissile isotope capable of sustaining a nuclear chain reaction, unlike uranium-238 which is fertile and must be converted into plutonium-239 to become fissile.
Fertile isotope
Uranium-238 is the primary fertile isotope that transforms into fissile plutonium-239 through neutron absorption in nuclear reactors.
Neutron capture
Uranium-235 exhibits a significantly higher neutron capture cross-section of approximately 584 barns compared to uranium-238's 2.7 barns, making it far more efficient for sustaining nuclear chain reactions.
Thermal reactor
Uranium-235, with its high neutron fission cross-section, is the primary fuel in thermal reactors, whereas uranium-238 mainly acts as a fertile material, absorbing neutrons to breed plutonium-239.
Fast breeder reactor
Uranium-235's high fissile properties enable fast breeder reactors to efficiently convert non-fissile uranium-238 into plutonium-239, maximizing fuel utilization compared to typical reactors.
Decay chain
The decay chain of uranium-235 involves a series of 11 alpha and beta decays leading to stable lead-207, while uranium-238 undergoes a longer decay chain of 14 steps ending in stable lead-206.
Cross-section (nuclear)
Uranium-235 has a significantly higher neutron-induced fission cross-section, approximately 584 barns for thermal neutrons, compared to uranium-238's much lower fast neutron absorption cross-section of about 2.7 barns, making U-235 far more efficient for sustaining nuclear chain reactions.
Nuclear fuel cycle
Uranium-235, with its fissile properties, plays a critical role in the nuclear fuel cycle by sustaining chain reactions in reactors, whereas uranium-238 serves primarily as fertile material that converts into plutonium-239 for subsequent fuel use.
Enrichment cascade
Uranium-235 is selectively concentrated in an enrichment cascade where hundreds of centrifuges or stages progressively increase its concentration from natural levels of 0.7% to weapons-grade levels above 90%, distinguishing it from the more abundant uranium-238.
uranium-235 vs uranium-238 Infographic
 njnir.com
njnir.com