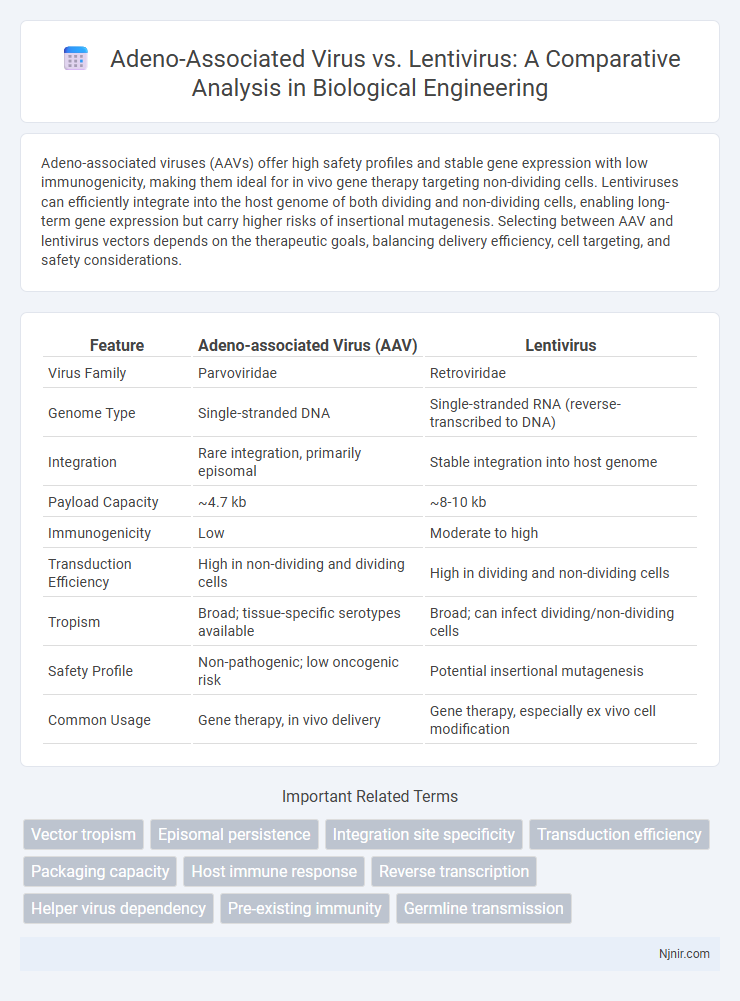
Adeno-associated viruses (AAVs) offer high safety profiles and stable gene expression with low immunogenicity, making them ideal for in vivo gene therapy targeting non-dividing cells. Lentiviruses can efficiently integrate into the host genome of both dividing and non-dividing cells, enabling long-term gene expression but carry higher risks of insertional mutagenesis. Selecting between AAV and lentivirus vectors depends on the therapeutic goals, balancing delivery efficiency, cell targeting, and safety considerations.
Table of Comparison
| Feature | Adeno-associated Virus (AAV) | Lentivirus |
|---|---|---|
| Virus Family | Parvoviridae | Retroviridae |
| Genome Type | Single-stranded DNA | Single-stranded RNA (reverse-transcribed to DNA) |
| Integration | Rare integration, primarily episomal | Stable integration into host genome |
| Payload Capacity | ~4.7 kb | ~8-10 kb |
| Immunogenicity | Low | Moderate to high |
| Transduction Efficiency | High in non-dividing and dividing cells | High in dividing and non-dividing cells |
| Tropism | Broad; tissue-specific serotypes available | Broad; can infect dividing/non-dividing cells |
| Safety Profile | Non-pathogenic; low oncogenic risk | Potential insertional mutagenesis |
| Common Usage | Gene therapy, in vivo delivery | Gene therapy, especially ex vivo cell modification |
Introduction to Viral Vectors in Gene Therapy
Adeno-associated virus (AAV) and lentivirus are prominent viral vectors used in gene therapy, each offering distinct advantages for gene delivery. AAV vectors are favored for their low immunogenicity and ability to transduce dividing and non-dividing cells, making them ideal for treating inherited diseases with long-term gene expression. Lentiviral vectors, derived from HIV, efficiently integrate into the host genome and are capable of delivering larger genetic payloads, which is critical for therapies targeting complex or large genes.
Overview of Adeno-Associated Virus (AAV)
Adeno-associated virus (AAV) is a small, non-enveloped single-stranded DNA virus widely used in gene therapy due to its low immunogenicity and ability to mediate long-term gene expression in non-dividing cells. AAV vectors exhibit limited packaging capacity of approximately 4.7 kb, but offer a favorable safety profile with minimal risk of insertional mutagenesis compared to lentivirus-based vectors. Its broad tissue tropism and stable episomal persistence make AAV an ideal choice for treating genetic disorders affecting the retina, liver, and central nervous system.
Overview of Lentivirus Vectors
Lentivirus vectors, derived from the HIV-1 virus, are widely used in gene therapy due to their ability to transduce both dividing and non-dividing cells with high efficiency. These vectors integrate into the host genome, enabling stable and long-term expression of therapeutic genes, which makes them ideal for treating genetic disorders and chronic diseases. Compared to adeno-associated virus (AAV) vectors, lentivirus vectors can carry larger transgenes up to 8-10 kb, providing greater versatility for delivering complex genetic payloads.
Genome Capacity and Genetic Payload
Adeno-associated virus (AAV) vectors have a genome capacity of approximately 4.7 kilobases, limiting their genetic payload to smaller genes or gene fragments. Lentiviral vectors offer a significantly larger genetic payload capacity, accommodating up to about 8-10 kilobases of genetic material. The choice between AAV and lentivirus vectors depends on the size of the therapeutic gene or transgene cassette required for effective gene delivery and expression.
Mechanisms of Cellular Entry and Integration
Adeno-associated virus (AAV) enters cells primarily through receptor-mediated endocytosis, utilizing specific cell surface receptors such as AAVR for attachment and internalization, followed by trafficking to the nucleus where it remains predominantly episomal with limited integration. Lentivirus vectors, derived from HIV-1, gain cellular entry via CD4 and chemokine coreceptors, leading to fusion at the plasma membrane and direct release of the viral core into the cytoplasm; lentiviral DNA is actively integrated into the host genome through the action of the viral integrase enzyme, enabling stable and long-term gene expression. The distinct integration profiles, with AAV favoring non-integrating episomal persistence and lentiviruses promoting efficient chromosomal integration, significantly influence their applications in gene therapy regarding safety and durability of transgene expression.
Transduction Efficiency and Target Cell Types
Adeno-associated virus (AAV) exhibits high transduction efficiency primarily in non-dividing cells and is widely used for gene delivery in neuronal and retinal cells due to its low immunogenicity and stable expression. Lentivirus demonstrates superior transduction efficiency in dividing and non-dividing cells, making it highly effective for gene transfer in hematopoietic stem cells, T cells, and other immune-related cells. The choice between AAV and lentivirus depends on target cell type specificity, with AAV favoring post-mitotic cells and lentivirus preferred for transducing a broader range of proliferating and non-proliferating cell types.
Immunogenicity and Safety Profiles
Adeno-associated viruses (AAVs) exhibit low immunogenicity and a strong safety profile due to their non-pathogenic nature and limited inflammatory response, making them ideal for in vivo gene therapy applications. Lentiviruses, derived from HIV-1, have moderate immunogenicity but can integrate into the host genome, raising concerns about insertional mutagenesis and long-term safety. Both vectors require careful consideration of immune responses, with AAVs generally preferred when minimizing immune activation and off-target effects is critical.
Long-term Expression and Stability
Adeno-associated virus (AAV) vectors provide stable, long-term gene expression with minimal immune response and low risk of insertional mutagenesis, making them ideal for non-dividing cells and in vivo applications. Lentivirus vectors integrate into the host genome, enabling sustained transgene expression in both dividing and non-dividing cells, but carry a higher risk of insertional mutagenesis and immune activation. For therapies requiring durable and stable gene expression, lentiviruses offer robust integration, whereas AAVs excel in safety and persistence without genomic integration.
Clinical Applications: AAV vs Lentivirus
Adeno-associated viruses (AAV) are widely favored in clinical applications for gene therapy due to their low immunogenicity and long-term gene expression in non-dividing cells, making them ideal for treating inherited retinal diseases and neurological disorders. Lentiviral vectors are preferred in clinical settings requiring stable integration into the host genome for durable gene expression, such as in hematopoietic stem cell therapies and CAR-T cell treatments. Both virus types show distinct advantages depending on the target tissue and desired duration of gene expression, influencing their application in personalized medicine approaches.
Future Perspectives in Viral Vector Development
Adeno-associated virus (AAV) vectors offer promising future perspectives due to their low immunogenicity and ability to mediate long-term gene expression in non-dividing cells, making them ideal for chronic diseases and inherited disorders. Lentiviral vectors, characterized by their capacity to integrate into the host genome and transduce dividing and non-dividing cells, remain crucial for stable gene therapy applications, especially in hematopoietic stem cells. Advances in genome editing and capsid engineering are expected to enhance specificity, reduce off-target effects, and improve safety profiles for both AAV and lentiviral systems, driving the next generation of viral vectors.
Vector tropism
Adeno-associated virus exhibits broad tissue tropism with distinct serotypes favoring specific cell types, while Lentivirus demonstrates efficient transduction of dividing and non-dividing cells, particularly in hematopoietic and central nervous system tissues.
Episomal persistence
Adeno-associated virus maintains therapeutic gene expression through stable episomal persistence avoiding genomic integration, whereas lentivirus integrates into the host genome enabling long-term expression but raising insertional mutagenesis risks.
Integration site specificity
Adeno-associated viruses primarily integrate into the AAVS1 site on chromosome 19 with high specificity, whereas lentiviruses exhibit random integration across gene-rich regions, increasing the risk of insertional mutagenesis.
Transduction efficiency
Lentivirus exhibits higher transduction efficiency than Adeno-associated virus, especially in non-dividing cells and large gene delivery applications.
Packaging capacity
Adeno-associated virus has a packaging capacity of approximately 4.7 kilobases, while lentivirus can package larger genetic payloads up to 8-10 kilobases.
Host immune response
Adeno-associated viruses (AAV) provoke a milder host immune response with limited inflammation compared to lentiviruses, which often trigger stronger adaptive immunity and higher risk of host immune-mediated clearance.
Reverse transcription
Adeno-associated virus undergoes single-stranded DNA conversion through host cell machinery instead of reverse transcription, while lentivirus reverse transcribes its RNA genome into DNA using viral reverse transcriptase before integration into the host genome.
Helper virus dependency
Adeno-associated virus (AAV) requires a helper virus such as adenovirus or herpesvirus for productive infection, whereas lentivirus can replicate independently without a helper virus.
Pre-existing immunity
Adeno-associated viruses face limited pre-existing immunity in humans compared to lentiviruses, which often encounter higher neutralizing antibody prevalence affecting gene therapy efficacy.
Germline transmission
Adeno-associated virus enables safer and more efficient germline transmission with lower insertional mutagenesis risk compared to lentivirus, which integrates more randomly and poses higher genotoxicity concerns.
Adeno-associated virus vs Lentivirus Infographic
 njnir.com
njnir.com