Lipofection uses lipid-based vesicles to encapsulate and deliver nucleic acids into cells by merging with the cell membrane, providing a gentle and efficient method suitable for a wide range of cell types. Electroporation employs short electrical pulses to transiently permeabilize the cell membrane, allowing direct entry of DNA, RNA, or proteins, which is particularly effective for hard-to-transfect cells or primary cells. Both techniques enhance gene delivery but vary in efficiency, cell type compatibility, and potential cytotoxicity, influencing their selection in biological engineering applications.
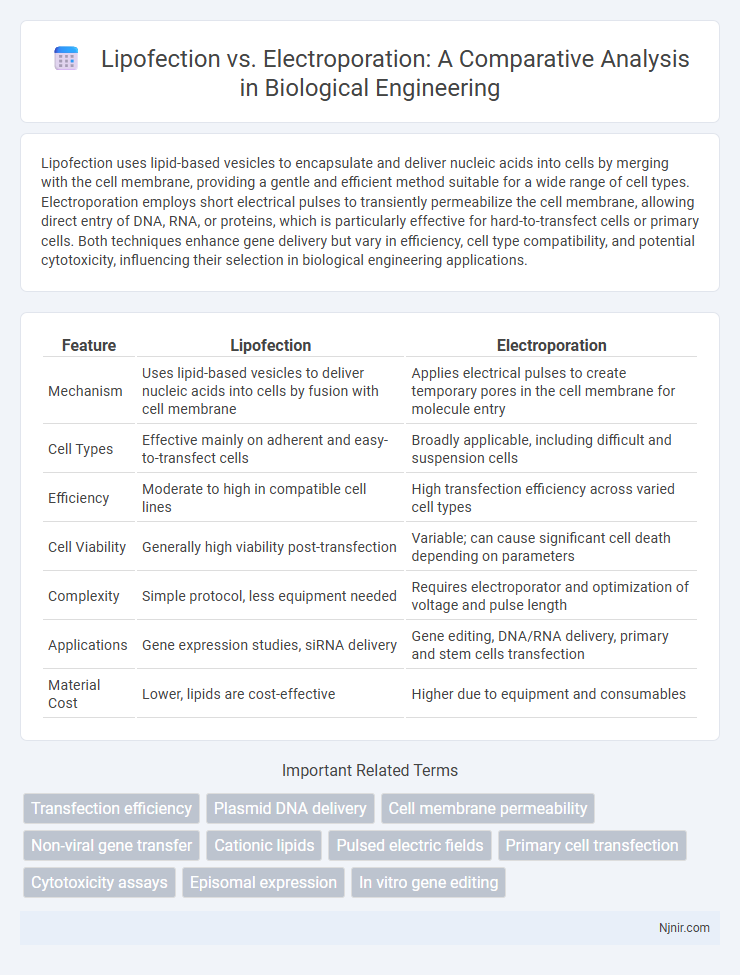
Table of Comparison
| Feature | Lipofection | Electroporation |
|---|---|---|
| Mechanism | Uses lipid-based vesicles to deliver nucleic acids into cells by fusion with cell membrane | Applies electrical pulses to create temporary pores in the cell membrane for molecule entry |
| Cell Types | Effective mainly on adherent and easy-to-transfect cells | Broadly applicable, including difficult and suspension cells |
| Efficiency | Moderate to high in compatible cell lines | High transfection efficiency across varied cell types |
| Cell Viability | Generally high viability post-transfection | Variable; can cause significant cell death depending on parameters |
| Complexity | Simple protocol, less equipment needed | Requires electroporator and optimization of voltage and pulse length |
| Applications | Gene expression studies, siRNA delivery | Gene editing, DNA/RNA delivery, primary and stem cells transfection |
| Material Cost | Lower, lipids are cost-effective | Higher due to equipment and consumables |
Introduction to Lipofection and Electroporation
Lipofection utilizes lipid-based vesicles to encapsulate and facilitate the delivery of nucleic acids into cells by merging with the lipid bilayer, ensuring efficient transfection with minimal cytotoxicity. Electroporation employs short electrical pulses to transiently permeabilize the cell membrane, enabling direct uptake of DNA, RNA, or proteins, but often requires optimization to balance transfection efficiency and cell viability. Both methods are pivotal in gene delivery, with lipofection favored for its biocompatibility and electroporation valued for its versatility across diverse cell types.
Mechanisms of DNA Delivery
Lipofection utilizes lipid-based vesicles to encapsulate DNA, facilitating its fusion with the cell membrane and subsequent release into the cytoplasm. Electroporation applies short electrical pulses to create transient pores in the cell membrane, allowing direct entry of DNA into the cytosol. Both methods aim to enhance cellular uptake of genetic material but differ fundamentally in membrane interaction and delivery efficiency.
Efficiency Comparison: Lipofection vs Electroporation
Lipofection demonstrates high transfection efficiency in a wide range of cell types, particularly effective in adherent mammalian cells due to its lipid-based delivery mechanism that facilitates membrane fusion. Electroporation offers superior efficiency for difficult-to-transfect cells, including primary and suspension cells, by temporarily creating pores in the cell membrane, enabling direct nucleic acid entry. Comparative studies reveal electroporation often achieves higher transfection rates in hard-to-transfect cells, while lipofection maintains better viability and consistency in commonly used cell lines.
Cell Type Compatibility and Versatility
Lipofection is highly compatible with a wide range of adherent and suspension cell types, including mammalian, insect, and plant cells, making it versatile for delivering nucleic acids into both easy-to-transfect and sensitive cell lines. Electroporation excels in transfecting difficult-to-transfect cells such as primary cells, stem cells, and immune cells by using electrical pulses to create transient pores in the cell membrane, but it may cause higher cell mortality depending on the cell type and pulse parameters. The choice between lipofection and electroporation depends on the specific cell type's membrane characteristics, transfection efficiency needs, and the balance between cell viability and versatility in experimental design.
Transfection Protocol Overview
Lipofection utilizes lipid-based reagents to form complexes with nucleic acids, facilitating their fusion with the cell membrane for efficient gene delivery, and is widely used for transfecting adherent cells in vitro. Electroporation employs controlled electrical pulses to create temporary pores in the cell membrane, allowing direct entry of DNA, RNA, or proteins into a variety of cell types, including difficult-to-transfect suspension cells. Optimization of parameters such as lipid-to-DNA ratio in lipofection and voltage, pulse duration, and number of pulses in electroporation is critical for maximizing transfection efficiency while minimizing cytotoxicity.
Impact on Cell Viability
Lipofection generally offers higher cell viability compared to electroporation due to its gentler membrane fusion mechanism, which reduces physical stress on cells. Electroporation employs electrical pulses that temporarily disrupt the cell membrane, often leading to increased cell death and reduced viability rates, especially in sensitive cell types. Optimizing voltage and pulse duration in electroporation can mitigate viability loss but typically does not match the biocompatibility level of lipofection.
Scalability for High-Throughput Applications
Lipofection offers moderate scalability for high-throughput applications due to its ease of use and compatibility with automated liquid handling systems, making it suitable for screening large sample numbers. Electroporation, while highly efficient for a diverse range of cell types, faces scalability challenges related to specialized equipment and optimization requirements for each cell line. Advances in multi-well electroporation devices are improving throughput capacity, but lipofection remains more readily adaptable for large-scale screening pipelines in drug discovery and genomics research.
Cost and Resource Considerations
Lipofection generally offers a cost-effective solution for transfection due to its lower equipment requirements and availability of commercial reagents, making it suitable for labs with limited budgets. Electroporation involves higher initial investment in specialized instruments and consumables, which can increase overall expenses despite its efficiency in delivering nucleic acids into difficult-to-transfect cells. Resource allocation for electroporation also includes ongoing maintenance and electricity costs, while lipofection mainly demands reagent replenishment and minimal setup.
Typical Applications in Biological Engineering
Lipofection is widely used for transfecting mammalian cells with nucleic acids in gene therapy research and protein expression studies due to its efficiency in delivering DNA, RNA, or siRNA into cultured cells. Electroporation is preferred for applications requiring the introduction of molecules into difficult-to-transfect cells, such as primary cells or stem cells, and is commonly employed in genetic engineering, CRISPR gene editing, and vaccine development. Both techniques are integral for delivering genetic material but vary in usage depending on cell type and experimental goals in biological engineering.
Future Directions and Emerging Trends
Future directions in Lipofection emphasize improved lipid formulations to enhance transfection efficiency and reduce cytotoxicity, enabling broader applications in gene therapy and mRNA vaccine delivery. Electroporation is evolving with precision-controlled pulse technologies and microfluidic platforms to increase cell viability and targeting specificity for clinical and research use. Emerging trends combine Lipofection and Electroporation with nanotechnology and CRISPR-based editing, driving advancements in personalized medicine and regenerative therapies.
Transfection efficiency
Lipofection typically achieves higher transfection efficiency in mammalian cells with minimal cytotoxicity compared to electroporation, which offers moderate efficiency but can cause significant cell damage.
Plasmid DNA delivery
Lipofection offers high-efficiency plasmid DNA delivery through lipid-based vesicles with low cytotoxicity, while electroporation utilizes electrical pulses to temporarily permeabilize cell membranes, enabling plasmid DNA uptake in a broader range of cell types but with increased cell stress.
Cell membrane permeability
Lipofection increases cell membrane permeability by encapsulating nucleic acids in liposomes for fusion with the lipid bilayer, whereas electroporation temporarily disrupts membrane integrity using electrical pulses to create pores for molecular entry.
Non-viral gene transfer
Lipofection offers a gentle, lipid-based non-viral gene transfer method ideal for transfecting sensitive cells, whereas electroporation uses electrical pulses to create temporary membrane pores, enabling efficient gene delivery in a broader range of cell types but with higher cytotoxicity.
Cationic lipids
Cationic lipids in lipofection facilitate efficient nucleic acid delivery by forming lipoplexes that enhance cellular uptake, whereas electroporation relies on electrical pulses to transiently permeabilize cell membranes without the use of lipid-based carriers.
Pulsed electric fields
Pulsed electric fields in electroporation create transient pores in cell membranes, enabling efficient transfection compared to lipofection's chemical lipid vesicles.
Primary cell transfection
Lipofection offers higher cell viability for primary cell transfection, while electroporation provides more efficient DNA delivery but with increased cell mortality.
Cytotoxicity assays
Lipofection generally demonstrates lower cytotoxicity in cytotoxicity assays compared to electroporation, which can cause significant cell membrane damage leading to higher cell death rates.
Episomal expression
Lipofection achieves higher episomal expression efficiency in mammalian cells compared to electroporation, which often results in increased cell mortality and lower transfection specificity.
In vitro gene editing
Lipofection offers gentle, efficient delivery of nucleic acids for in vitro gene editing with high cell viability, while electroporation provides robust transfection efficiency across diverse cell types at the cost of increased cellular stress and potential damage.
Lipofection vs Electroporation Infographic
 njnir.com
njnir.com