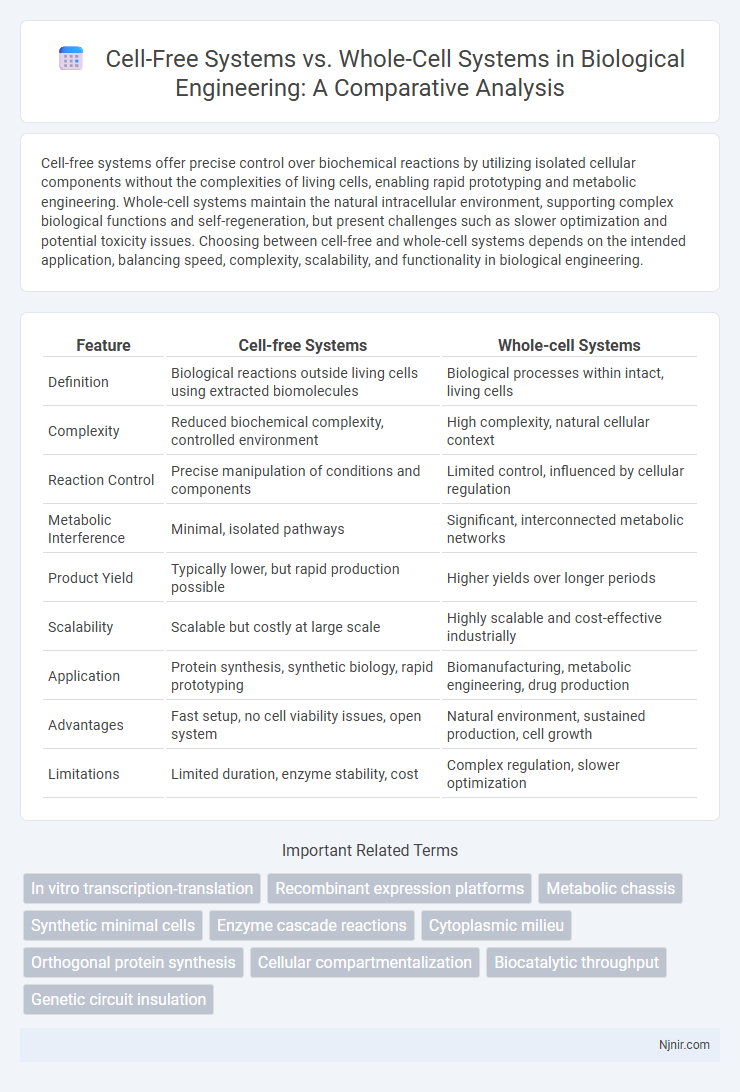
Cell-free systems offer precise control over biochemical reactions by utilizing isolated cellular components without the complexities of living cells, enabling rapid prototyping and metabolic engineering. Whole-cell systems maintain the natural intracellular environment, supporting complex biological functions and self-regeneration, but present challenges such as slower optimization and potential toxicity issues. Choosing between cell-free and whole-cell systems depends on the intended application, balancing speed, complexity, scalability, and functionality in biological engineering.
Table of Comparison
| Feature | Cell-free Systems | Whole-cell Systems |
|---|---|---|
| Definition | Biological reactions outside living cells using extracted biomolecules | Biological processes within intact, living cells |
| Complexity | Reduced biochemical complexity, controlled environment | High complexity, natural cellular context |
| Reaction Control | Precise manipulation of conditions and components | Limited control, influenced by cellular regulation |
| Metabolic Interference | Minimal, isolated pathways | Significant, interconnected metabolic networks |
| Product Yield | Typically lower, but rapid production possible | Higher yields over longer periods |
| Scalability | Scalable but costly at large scale | Highly scalable and cost-effective industrially |
| Application | Protein synthesis, synthetic biology, rapid prototyping | Biomanufacturing, metabolic engineering, drug production |
| Advantages | Fast setup, no cell viability issues, open system | Natural environment, sustained production, cell growth |
| Limitations | Limited duration, enzyme stability, cost | Complex regulation, slower optimization |
Introduction to Cell-Free and Whole-Cell Systems
Cell-free systems utilize cellular extracts or purified components to perform biological reactions outside living cells, enabling precise control of experimental conditions and rapid protein synthesis. Whole-cell systems involve intact living cells that carry out complex metabolic processes, offering naturally optimized environments for enzyme expression and regulation. These platforms differ in scalability, experimental flexibility, and applications across synthetic biology and biomanufacturing.
Fundamental Principles of Cell-Free Systems
Cell-free systems rely on the extraction of cellular machinery, such as ribosomes, tRNAs, and enzymes, to perform protein synthesis outside living cells, enabling precise control over biochemical reactions without cell membrane constraints. These systems facilitate direct manipulation of reaction conditions, genetic templates, and cofactors, enhancing flexibility and rapid prototyping in synthetic biology and protein engineering. Unlike whole-cell systems that depend on intact cellular metabolism and growth, cell-free platforms allow for high-throughput protein expression and pathway optimization with reduced interference from cellular regulation.
Core Mechanisms of Whole-Cell Systems
Whole-cell systems rely on living microorganisms that maintain intact cellular structures and metabolic networks, enabling complex biochemical pathways and regulation mechanisms. Core mechanisms include gene expression controlled by native transcription and translation machinery, coupled with cellular metabolism that supplies energy and precursors for biosynthesis. This integrated environment supports dynamic responses to environmental changes and self-regulation, unlike isolated cell-free systems.
Comparative Analysis: Flexibility and Customization
Cell-free systems offer superior flexibility and customization compared to whole-cell systems by allowing direct manipulation of biochemical components without cellular constraints. They enable precise control over reaction conditions and facilitate the incorporation of non-natural amino acids or synthetic pathways. Whole-cell systems, while more robust for complex metabolic processes, have limited modulation capabilities due to cellular regulatory mechanisms and membrane transport barriers.
Efficiency in Protein Synthesis: Cell-Free vs Whole-Cell
Cell-free systems offer higher efficiency in protein synthesis by enabling direct control over transcription and translation processes without cellular constraints, resulting in faster and more accurate protein production. Whole-cell systems, while capable of post-translational modifications and complex folding, often face limitations from cellular metabolism and resource allocation, which can reduce yield and speed. Optimization of cell-free platforms enhances scalability and reduces synthesis time, making them superior for rapid protein expression in research and industrial applications.
Scalability and Industrial Applications
Cell-free systems offer superior scalability with rapid reaction times and easy control over reaction conditions, making them ideal for high-throughput synthesis and customizable biomanufacturing processes. Whole-cell systems provide robust metabolic networks and self-replicating capabilities that support large-scale, continuous production in industrial biotechnology such as pharmaceuticals, biofuels, and enzyme manufacturing. The choice between cell-free and whole-cell systems depends on the specific industrial application, balancing efficiency, cost, and flexibility for optimal production outcomes.
Safety and Contamination Control
Cell-free systems minimize contamination risks by eliminating living organisms, reducing the potential for pathogenic growth and biohazardous waste. Whole-cell systems pose higher safety concerns due to cellular metabolism producing unpredictable byproducts and potential genetic mutations that could lead to contamination. Implementing strict sterile techniques and containment protocols is critical for whole-cell systems to maintain biosafety and prevent cross-contamination in bioprocessing environments.
Resource Utilization and Cost Considerations
Cell-free systems offer precise control over resource allocation by utilizing only essential biomolecular components, reducing waste and enhancing efficiency compared to whole-cell systems that require maintaining entire metabolic networks. Whole-cell systems involve higher operational costs due to complex nutrient requirements, prolonged incubation times, and cellular maintenance energy consumption. The streamlined nature of cell-free platforms leads to lower reagent expenses and faster production cycles, making them more cost-effective for synthetic biology and protein synthesis applications.
Innovative Applications in Synthetic Biology
Cell-free systems enable precise control over biochemical reactions by isolating transcription and translation machinery, facilitating rapid prototyping of genetic circuits and metabolic pathways in synthetic biology. Whole-cell systems offer complex intracellular environments that support long-term expression and functional integration of synthetic constructs, making them ideal for applications like bioproduction and environmental sensing. Advances in cell-free technology accelerate the design-build-test cycle, while whole-cell systems provide robustness necessary for scalable industrial and therapeutic uses.
Future Perspectives and Emerging Trends
Cell-free systems are revolutionizing synthetic biology by enabling faster prototyping, precise control of biochemical reactions, and the synthesis of non-natural biomolecules that whole-cell systems cannot efficiently produce. Advances in microfluidics, machine learning integration, and cost reduction are driving the scalability and commercial viability of cell-free platforms for therapeutics, biosensing, and biomanufacturing. Emerging trends emphasize hybrid approaches combining cell-free and whole-cell systems to leverage the robustness of living cells alongside the flexibility and modularity of cell-free expression for complex biochemical engineering.
In vitro transcription-translation
Cell-free systems enable rapid and controllable in vitro transcription-translation by directly utilizing cellular machinery outside living cells, whereas whole-cell systems rely on intact cellular processes, resulting in slower and less predictable protein synthesis.
Recombinant expression platforms
Cell-free systems enable rapid, controllable recombinant expression with reduced cellular complexity and toxicity, while whole-cell systems offer robust, scalable protein production leveraging native cellular machinery.
Metabolic chassis
Cell-free systems offer precise control over metabolic pathways and faster prototyping compared to whole-cell systems, which provide robust metabolic chassis with native regulatory networks and complex intracellular environments essential for scalable bioproduction.
Synthetic minimal cells
Synthetic minimal cells in cell-free systems enable precise control over gene expression and metabolic pathways unlike whole-cell systems, which involve complex cellular environments and regulatory networks.
Enzyme cascade reactions
Cell-free systems enable precise control and optimization of enzyme cascade reactions by isolating enzymatic components, whereas whole-cell systems offer natural cellular environments that support complex metabolic pathways but may present challenges like substrate competition and regulatory constraints.
Cytoplasmic milieu
Cell-free systems replicate the cytoplasmic milieu by providing a controlled environment with essential enzymes, ribosomes, and metabolites, enabling precise manipulation of biochemical reactions unlike whole-cell systems where complex intracellular interactions and regulatory mechanisms can limit experimental control.
Orthogonal protein synthesis
Orthogonal protein synthesis in cell-free systems enables precise control and incorporation of non-natural amino acids without cellular toxicity, surpassing whole-cell systems' limitations in genetic code expansion and protein engineering.
Cellular compartmentalization
Cell-free systems lack cellular compartmentalization, allowing direct access to biomolecular machinery and precise control over biochemical reactions, whereas whole-cell systems maintain compartmentalization that supports complex metabolic interactions but limits experimental manipulation.
Biocatalytic throughput
Cell-free systems achieve higher biocatalytic throughput than whole-cell systems by enabling direct enzyme access, faster reaction rates, and simplified metabolic control.
Genetic circuit insulation
Cell-free systems offer superior genetic circuit insulation compared to whole-cell systems by minimizing host cellular interference and providing a controlled environment for precise gene expression.
Cell-free systems vs Whole-cell systems Infographic
 njnir.com
njnir.com