Phage therapy offers a targeted approach by using bacteriophages to selectively infect and destroy antibiotic-resistant bacteria, reducing collateral damage to beneficial microbiota. Antibiotic therapy, while broad-spectrum and effective for many infections, often leads to resistance development and disrupts the microbiome balance. Advances in biological engineering enhance phage therapy's precision and potential as a complementary or alternative treatment to traditional antibiotics.
Table of Comparison
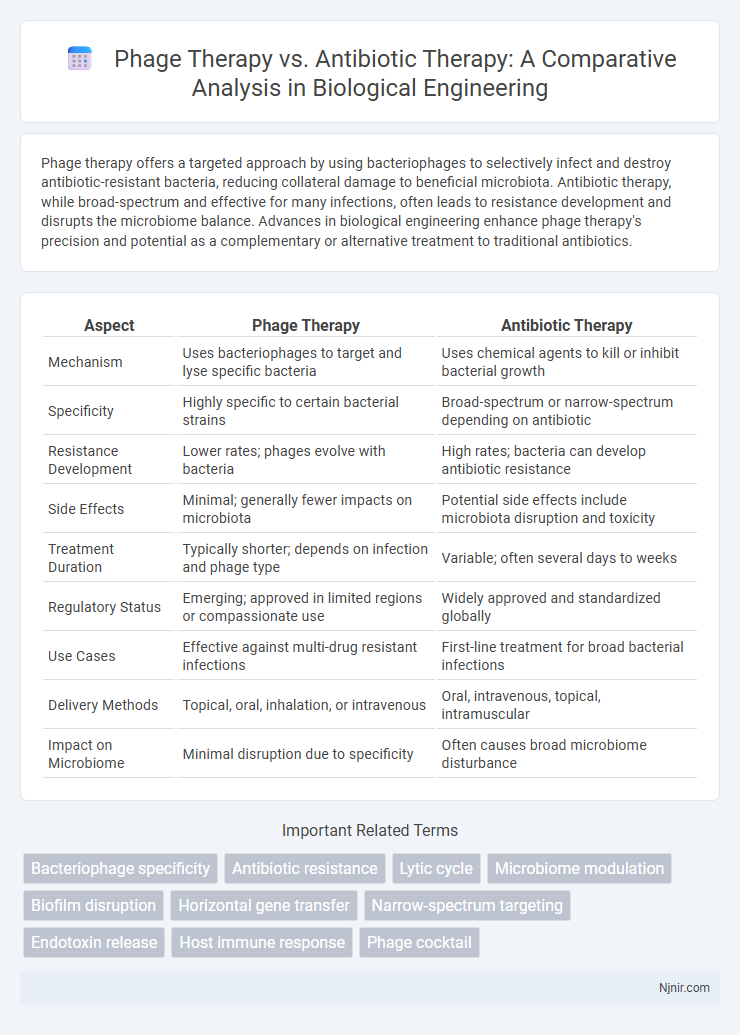
| Aspect | Phage Therapy | Antibiotic Therapy |
|---|---|---|
| Mechanism | Uses bacteriophages to target and lyse specific bacteria | Uses chemical agents to kill or inhibit bacterial growth |
| Specificity | Highly specific to certain bacterial strains | Broad-spectrum or narrow-spectrum depending on antibiotic |
| Resistance Development | Lower rates; phages evolve with bacteria | High rates; bacteria can develop antibiotic resistance |
| Side Effects | Minimal; generally fewer impacts on microbiota | Potential side effects include microbiota disruption and toxicity |
| Treatment Duration | Typically shorter; depends on infection and phage type | Variable; often several days to weeks |
| Regulatory Status | Emerging; approved in limited regions or compassionate use | Widely approved and standardized globally |
| Use Cases | Effective against multi-drug resistant infections | First-line treatment for broad bacterial infections |
| Delivery Methods | Topical, oral, inhalation, or intravenous | Oral, intravenous, topical, intramuscular |
| Impact on Microbiome | Minimal disruption due to specificity | Often causes broad microbiome disturbance |
Introduction to Phage and Antibiotic Therapies
Phage therapy utilizes bacteriophages, viruses that specifically infect and lyse bacteria, offering targeted treatment against antibiotic-resistant bacterial strains. Antibiotic therapy involves chemical agents that inhibit bacterial growth or kill bacteria broadly, but rising antibiotic resistance challenges its effectiveness. Phage therapy's specificity and ability to evolve alongside bacteria present promising alternatives to traditional antibiotics in combating multidrug-resistant infections.
Mechanisms of Action: Phages vs Antibiotics
Phage therapy employs bacteriophages that specifically infect and lyse bacterial cells by injecting their genetic material and hijacking the bacterial machinery to replicate, leading to targeted bacterial destruction without harming beneficial microbiota. Antibiotic therapy uses chemical agents that inhibit bacterial processes such as cell wall synthesis, protein production, or DNA replication, often resulting in broad-spectrum effects that can disrupt the entire microbial community. Unlike antibiotics, phages evolve alongside bacteria, reducing the risk of resistance and enabling a precise mechanism of bacterial eradication.
Historical Overview of Antimicrobial Strategies
Phage therapy, discovered by Felix d'Herelle in the early 20th century, offered a targeted approach to bacterial infections before antibiotics became widespread in the 1940s. Antibiotic therapy revolutionized antimicrobial treatment with the discovery of penicillin, leading to mass production and broad-spectrum applications that overshadowed phage therapy for decades. Renewed interest in phage therapy arises today from escalating antibiotic resistance and the need for alternative or complementary antimicrobial strategies.
Spectrum of Activity: Specificity and Resistance
Phage therapy offers a highly specific spectrum of activity by targeting only particular bacterial strains, minimizing disruption to beneficial microbiota and reducing the likelihood of resistance development. In contrast, antibiotic therapy typically exhibits broad-spectrum activity, which can eradicate a wide range of bacteria but often leads to significant microbiome imbalance and accelerates the emergence of multidrug-resistant pathogens. The precision of phage therapy makes it a promising alternative for combating antibiotic-resistant infections while preserving microbial diversity.
Efficacy in Treating Bacterial Infections
Phage therapy demonstrates high efficacy in treating antibiotic-resistant bacterial infections by specifically targeting and lysing pathogenic bacteria, reducing the risk of disruption to the beneficial microbiota. Antibiotic therapy remains effective for many common bacterial infections but faces challenges from increasing antibiotic resistance and broad-spectrum impacts on microbiome health. Recent studies highlight that phage therapy can serve as a complementary or alternative treatment in cases where antibiotics fail due to resistance mechanisms like biofilm formation or multidrug efflux pumps.
Development of Resistance: Challenges and Solutions
Phage therapy offers a targeted approach that significantly reduces the likelihood of developing bacterial resistance compared to broad-spectrum antibiotic therapy, which often drives multidrug-resistant strains. However, bacteria can still evolve resistance to bacteriophages, necessitating the development of phage cocktails and personalized phage formulations to overcome these challenges. Advances in genetic engineering and rapid phage screening techniques facilitate the creation of adaptable phage therapies, enhancing their effectiveness against resistant bacterial populations.
Safety Profiles and Side Effects
Phage therapy demonstrates a favorable safety profile, with minimal side effects such as mild inflammation or allergic reactions, due to its specificity in targeting bacterial pathogens without harming beneficial microbiota. In contrast, antibiotic therapy often presents broader systemic side effects, including gastrointestinal disturbances, allergic reactions, and the risk of promoting antibiotic resistance. The precision of phage therapy reduces the incidence of dysbiosis and toxicity, making it a safer alternative for treating multidrug-resistant bacterial infections.
Regulatory and Manufacturing Considerations
Phage therapy faces unique regulatory challenges due to its use of live viruses, requiring tailored guidelines for safety, efficacy, and quality control distinct from those governing antibiotic therapies. Manufacturing of bacteriophages demands stringent purification processes to eliminate endotoxins and ensure consistent potency, contrasting with the chemical synthesis and scalability of antibiotics. Regulatory agencies are increasingly developing frameworks to address the personalized nature of phage therapy while maintaining rigorous standards akin to established antibiotic approval pathways.
Current Clinical Applications and Case Studies
Phage therapy demonstrates targeted eradication of antibiotic-resistant bacteria in clinical trials, notably treating chronic Pseudomonas aeruginosa infections in cystic fibrosis patients. Recent case studies reveal successful use of bacteriophages against multidrug-resistant Acinetobacter baumannii bloodstream infections where antibiotics failed. Antibiotic therapy remains widespread but faces increasing limitations due to resistance, driving renewed interest in phage applications for persistent and drug-resistant bacterial diseases.
Future Prospects in Biological Engineering Therapies
Phage therapy offers a promising alternative to antibiotic therapy by targeting specific bacterial pathogens with minimal impact on beneficial microbiota, addressing the rise of antibiotic resistance. Advances in genetic engineering enable the customization of bacteriophages to enhance efficacy, deliver antimicrobial payloads, and overcome bacterial defense mechanisms. Future prospects in biological engineering therapies include integrating synthetic biology and CRISPR technologies to develop sophisticated phage-based treatments that could revolutionize infectious disease management.
Bacteriophage specificity
Phage therapy targets bacterial infections with high specificity by using bacteriophages that infect only particular bacterial strains, unlike broad-spectrum antibiotic therapy that affects a wide range of bacteria.
Antibiotic resistance
Phage therapy offers a targeted alternative to antibiotic therapy, effectively combating bacterial infections while reducing the risk of antibiotic resistance development.
Lytic cycle
Phage therapy targets bacterial infections by utilizing bacteriophages in the lytic cycle to specifically infect and lyse bacteria, offering a precise alternative to broad-spectrum antibiotic therapy that often leads to resistance.
Microbiome modulation
Phage therapy selectively targets pathogenic bacteria, preserving beneficial microbiome diversity, whereas antibiotic therapy often disrupts microbial balance by broadly eliminating both harmful and beneficial bacteria.
Biofilm disruption
Phage therapy effectively disrupts biofilms by targeting and degrading extracellular polymeric substances, enhancing bacterial eradication compared to traditional antibiotic therapy.
Horizontal gene transfer
Phage therapy reduces the risk of horizontal gene transfer compared to antibiotic therapy, which often promotes the spread of antibiotic resistance genes among bacterial populations.
Narrow-spectrum targeting
Phage therapy offers narrow-spectrum targeting by precisely attacking specific bacterial strains, reducing collateral damage to beneficial microbiota compared to broad-spectrum antibiotic therapy.
Endotoxin release
Phage therapy significantly reduces endotoxin release compared to antibiotic therapy by selectively targeting and lysing bacterial cells without causing widespread bacterial cell lysis that triggers endotoxin surge.
Host immune response
Phage therapy enhances host immune response by specifically targeting bacterial pathogens while minimizing disruption to beneficial microbiota, unlike antibiotic therapy which often triggers broad immune reactions and resistance development.
Phage cocktail
Phage cocktail therapy offers targeted bacterial eradication by combining multiple bacteriophages to combat antibiotic-resistant infections, enhancing treatment specificity and reducing the risk of bacterial resistance compared to traditional antibiotic therapy.
Phage therapy vs Antibiotic therapy Infographic
 njnir.com
njnir.com