Microbiome manipulation offers precise modulation of microbial communities by targeting specific genes or metabolic pathways, enhancing host health and disease resistance more effectively than general probiotic therapy. Probiotic therapy introduces beneficial microorganisms but often lacks the ability to integrate or adapt within the existing microbiome ecosystem, limiting its long-term efficacy. Advanced techniques in microbiome engineering enable tailored interventions that surpass conventional probiotics by fostering stable and functional microbial consortia.
Table of Comparison
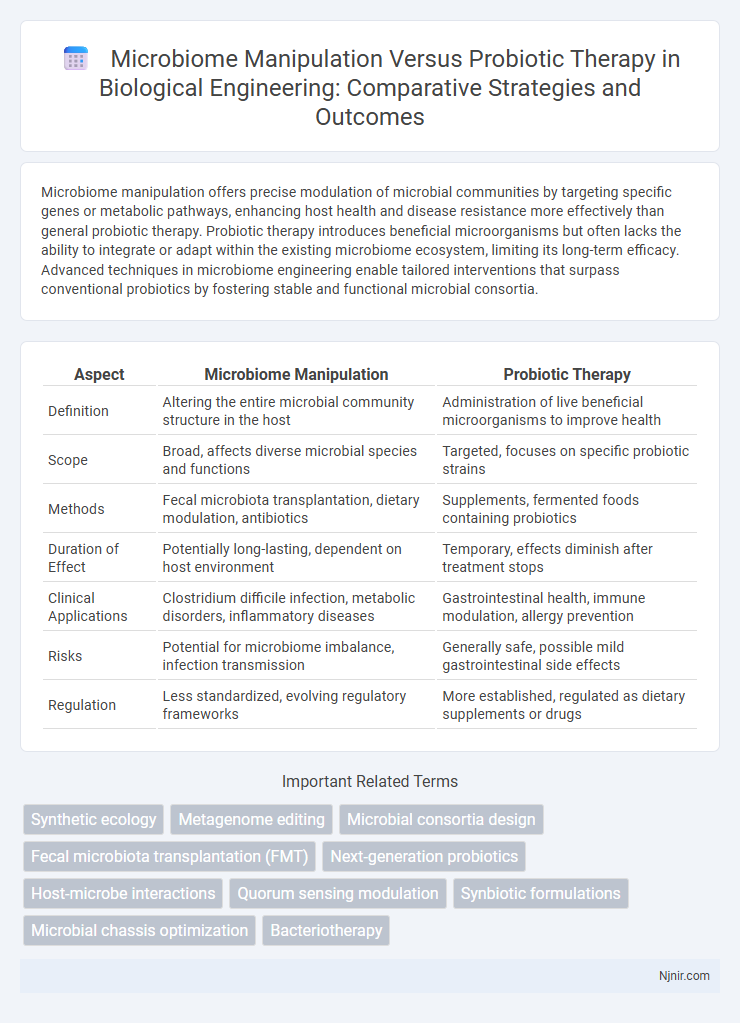
| Aspect | Microbiome Manipulation | Probiotic Therapy |
|---|---|---|
| Definition | Altering the entire microbial community structure in the host | Administration of live beneficial microorganisms to improve health |
| Scope | Broad, affects diverse microbial species and functions | Targeted, focuses on specific probiotic strains |
| Methods | Fecal microbiota transplantation, dietary modulation, antibiotics | Supplements, fermented foods containing probiotics |
| Duration of Effect | Potentially long-lasting, dependent on host environment | Temporary, effects diminish after treatment stops |
| Clinical Applications | Clostridium difficile infection, metabolic disorders, inflammatory diseases | Gastrointestinal health, immune modulation, allergy prevention |
| Risks | Potential for microbiome imbalance, infection transmission | Generally safe, possible mild gastrointestinal side effects |
| Regulation | Less standardized, evolving regulatory frameworks | More established, regulated as dietary supplements or drugs |
Introduction to Microbiome Manipulation and Probiotic Therapy
Microbiome manipulation involves altering the complex community of microorganisms in the human gut to improve health outcomes, utilizing methods such as fecal microbiota transplantation, dietary changes, and targeted antibiotics. Probiotic therapy specifically introduces live beneficial bacteria strains, like Lactobacillus and Bifidobacterium, to restore microbial balance and enhance immune function. Both strategies aim to influence gut microbiota composition but differ in scope, precision, and application techniques.
Defining the Human Microbiome
The human microbiome consists of trillions of microorganisms residing in and on the body, playing a crucial role in health and disease. Microbiome manipulation involves altering the entire microbial community to restore balance, while probiotic therapy introduces specific beneficial strains to support gut health. Understanding the complexity and diversity of the microbiome is essential for developing targeted approaches in microbiome-based treatments.
Probiotics: Mechanisms of Action
Probiotic therapy exerts its beneficial effects primarily through mechanisms such as competitive exclusion of pathogens, enhancement of gut barrier function, and modulation of host immune responses. Specific probiotic strains produce antimicrobial substances like bacteriocins and short-chain fatty acids that inhibit harmful microbes and support a balanced microbiome. These actions collectively promote gastrointestinal health, reduce inflammation, and improve metabolic homeostasis within the host.
Microbiome Manipulation Techniques
Microbiome manipulation techniques encompass targeted interventions such as fecal microbiota transplantation (FMT), prebiotic supplementation, and synbiotic formulations designed to alter the gut microbial composition for therapeutic benefits. Unlike traditional probiotic therapy, which introduces specific beneficial bacterial strains, microbiome manipulation focuses on reshaping the entire microbial ecosystem to restore balance and enhance metabolic functions. Advanced methods include phage therapy and CRISPR-based gene editing to selectively modulate microbial populations, offering precision tools for treating dysbiosis-related diseases.
Comparative Efficacy in Disease Modulation
Microbiome manipulation through fecal microbiota transplantation (FMT) demonstrates broader and more sustained shifts in gut microbial diversity compared to probiotic therapy, which typically introduces limited strains with transient colonization. Clinical studies reveal that FMT shows superior efficacy in treating recurrent Clostridioides difficile infections and emerging potential in inflammatory bowel disease, whereas probiotics primarily aid in mild gastrointestinal symptoms and preventive care. The comprehensive ecological restoration from microbiome manipulation contrasts with the targeted but narrower benefits of probiotic supplementation in disease modulation.
Safety and Regulatory Considerations
Microbiome manipulation techniques, such as fecal microbiota transplantation (FMT) and targeted bacteriophage therapy, pose unique safety challenges including the risk of pathogen transfer and immune reactions, necessitating rigorous screening and monitoring protocols. Probiotic therapy, often involving well-characterized bacterial strains like Lactobacillus and Bifidobacterium, tends to have a more established safety profile but still requires regulation to prevent contamination and ensure strain efficacy. Regulatory agencies like the FDA and EMA emphasize strict guidelines for both approaches, focusing on product consistency, clinical evidence, and patient safety to minimize adverse effects and enhance therapeutic outcomes.
Advances in Precision Microbiome Engineering
Advances in precision microbiome engineering enable targeted manipulation of microbial communities, surpassing traditional probiotic therapies by customizing interventions at the genetic and functional levels. Techniques such as CRISPR-based editing and synthetic biology facilitate the design of microbial consortia tailored to restore gut homeostasis with higher specificity and efficacy. These innovations drive personalized microbiome treatments that improve outcomes in metabolic, inflammatory, and infectious diseases through precise modulation of microbial pathways.
Challenges in Clinical Translation
Microbiome manipulation faces challenges in clinical translation due to the complex and individualized nature of microbial ecosystems, making standardized treatments difficult to develop. Probiotic therapy struggles with inconsistent efficacy, limited strain-specific evidence, and regulatory constraints that hinder large-scale adoption. Both approaches require advanced biomarker identification and personalized strategies to improve safety, reproducibility, and therapeutic outcomes in clinical settings.
Future Directions in Microbiome-Based Interventions
Future directions in microbiome-based interventions emphasize precision microbiome manipulation techniques such as phage therapy, targeted prebiotics, and CRISPR-based editing to customize microbial communities for specific health outcomes. Advances in multi-omics technologies and artificial intelligence are enabling personalized probiotic therapies that enhance efficacy by tailoring strains to individual microbiome profiles. Integration of synthetic biology with probiotic development promises novel therapeutic options with improved safety, stability, and functionality for treating complex diseases.
Integrating Microbiome Science into Personalized Medicine
Microbiome manipulation leverages advanced genomic and metabolomic profiling to tailor interventions that modify microbial communities precisely, optimizing patient-specific outcomes in personalized medicine. Probiotic therapy introduces beneficial microbial strains but often lacks the specificity achieved through comprehensive microbiome analysis, limiting its efficacy in individualized treatment plans. Integrating microbiome science into personalized medicine enables dynamic modulation of host-microbiome interactions, enhancing therapeutic precision and reducing adverse effects.
Synthetic ecology
Synthetic ecology leverages engineered microbial consortia to precisely manipulate the microbiome, offering targeted advantages over traditional probiotic therapy by enhancing microbial interactions and ecosystem stability.
Metagenome editing
Metagenome editing enables precise microbiome manipulation by directly altering microbial genes, offering more targeted and sustainable therapeutic effects compared to traditional probiotic therapy.
Microbial consortia design
Microbial consortia design enables precise microbiome manipulation by engineering synergistic microbial communities, offering targeted and sustainable therapeutic benefits compared to traditional single-strain probiotic therapy.
Fecal microbiota transplantation (FMT)
Fecal microbiota transplantation (FMT) offers a more comprehensive and effective microbiome manipulation approach than traditional probiotic therapy by restoring gut microbial diversity and function through direct transfer of healthy donor fecal matter.
Next-generation probiotics
Next-generation probiotics leverage precise microbiome manipulation techniques to enhance targeted therapeutic outcomes beyond traditional probiotic therapy.
Host-microbe interactions
Microbiome manipulation strategically alters host-microbe interactions at a systemic level, whereas probiotic therapy introduces specific microbial strains to modulate localized host immune responses.
Quorum sensing modulation
Microbiome manipulation through targeted quorum sensing modulation offers a more precise method for regulating microbial communication and restoring balance compared to conventional probiotic therapy.
Synbiotic formulations
Synbiotic formulations combine prebiotics and probiotics to enhance microbiome manipulation by improving gut microbial balance and therapeutic efficacy compared to probiotic therapy alone.
Microbial chassis optimization
Microbial chassis optimization enhances microbiome manipulation by engineering robust microbial strains with tailored metabolic pathways for improved therapeutic efficacy compared to traditional probiotic therapy.
Bacteriotherapy
Bacteriotherapy, a targeted form of microbiome manipulation, uses specific bacterial strains to restore gut health more effectively than broad-spectrum probiotic therapy.
Microbiome manipulation vs Probiotic therapy Infographic
 njnir.com
njnir.com