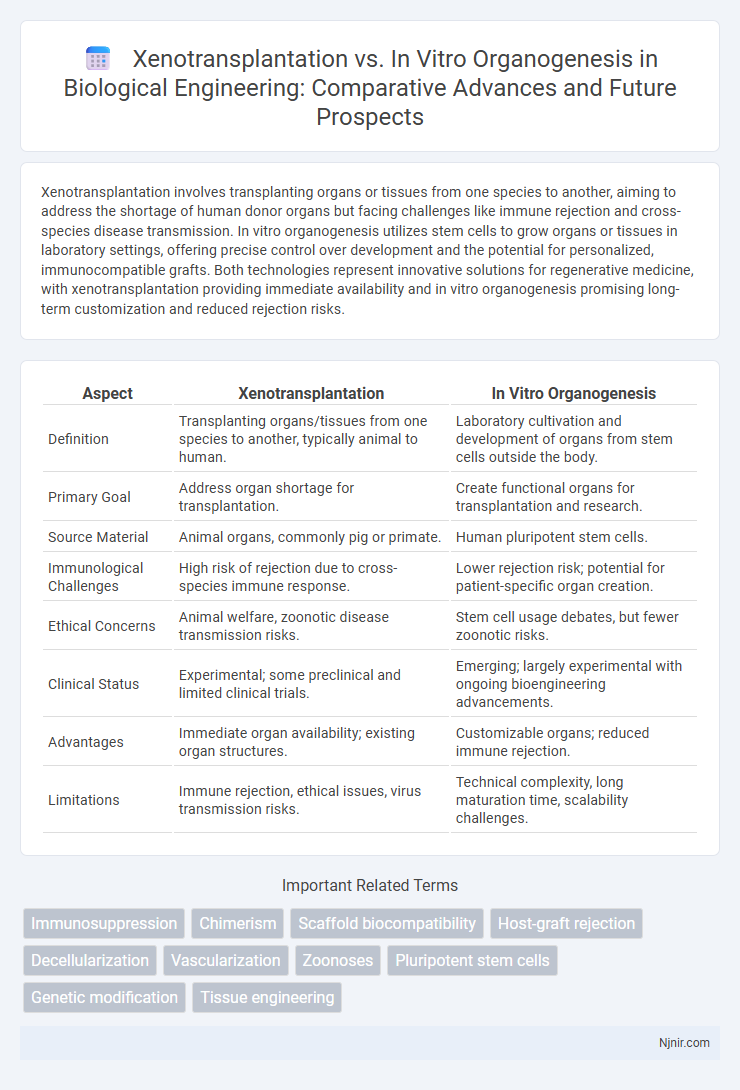
Xenotransplantation involves transplanting organs or tissues from one species to another, aiming to address the shortage of human donor organs but facing challenges like immune rejection and cross-species disease transmission. In vitro organogenesis utilizes stem cells to grow organs or tissues in laboratory settings, offering precise control over development and the potential for personalized, immunocompatible grafts. Both technologies represent innovative solutions for regenerative medicine, with xenotransplantation providing immediate availability and in vitro organogenesis promising long-term customization and reduced rejection risks.
Table of Comparison
| Aspect | Xenotransplantation | In Vitro Organogenesis |
|---|---|---|
| Definition | Transplanting organs/tissues from one species to another, typically animal to human. | Laboratory cultivation and development of organs from stem cells outside the body. |
| Primary Goal | Address organ shortage for transplantation. | Create functional organs for transplantation and research. |
| Source Material | Animal organs, commonly pig or primate. | Human pluripotent stem cells. |
| Immunological Challenges | High risk of rejection due to cross-species immune response. | Lower rejection risk; potential for patient-specific organ creation. |
| Ethical Concerns | Animal welfare, zoonotic disease transmission risks. | Stem cell usage debates, but fewer zoonotic risks. |
| Clinical Status | Experimental; some preclinical and limited clinical trials. | Emerging; largely experimental with ongoing bioengineering advancements. |
| Advantages | Immediate organ availability; existing organ structures. | Customizable organs; reduced immune rejection. |
| Limitations | Immune rejection, ethical issues, virus transmission risks. | Technical complexity, long maturation time, scalability challenges. |
Introduction to Xenotransplantation and In Vitro Organogenesis
Xenotransplantation involves the transplantation of organs or tissues between different species, primarily using genetically modified animal organs such as pig hearts to address the shortage of human donor organs. In vitro organogenesis refers to the laboratory cultivation and development of organs from stem cells or progenitor cells, aiming to create fully functional organs for transplantation. Both approaches seek to overcome organ scarcity but differ fundamentally in their biological sources and methodologies.
Historical Background and Development
Xenotransplantation has evolved from early 20th-century experiments involving animal-to-human organ and tissue transfers, with significant milestones including the 1960s baboon-to-human heart transplant attempts and recent genetic modifications to reduce immunogenicity in pig organs. In vitro organogenesis, rooted in advances in stem cell biology since the late 1990s, has progressed through the development of organoids and bioengineered tissues that mimic native organ structures and functions within controlled laboratory environments. Both fields have rapidly advanced due to improvements in immunology, genetic engineering, and biomaterials, shaping contemporary regenerative medicine and transplantation strategies.
Scientific Principles Underpinning Each Approach
Xenotransplantation relies on immunological compatibility and genetic engineering to modify animal organs, primarily from pigs, to reduce rejection risks when transplanted into humans. In vitro organogenesis employs stem cell biology and tissue engineering techniques to create functional human organs by guiding pluripotent stem cells through developmental pathways in a controlled laboratory environment. Both approaches depend heavily on advancements in cellular biology and immunology to overcome barriers in organ availability and transplant rejection.
Major Advancements and Milestones
Xenotransplantation has achieved major milestones with the successful genetic modification of pigs to reduce immune rejection, leading to prolonged survival of transplanted organs in primate models. In vitro organogenesis advances include the creation of functional mini-organs from stem cells, such as lab-grown kidneys and hearts, demonstrating potential for personalized regenerative medicine. Both fields have made significant strides in overcoming immunological barriers and improving organ viability, positioning them as promising solutions to the organ shortage crisis.
Source Materials: Animal Donors vs. Stem Cell Cultures
Xenotransplantation relies on animal donors, primarily genetically modified pigs, to provide whole organs or tissues compatible with human recipients, aiming to address organ shortages. In vitro organogenesis utilizes stem cell cultures, especially pluripotent stem cells and induced pluripotent stem cells (iPSCs), to grow functional organoids or tissues in laboratory settings, offering personalized and immunocompatible transplantation options. The choice of source materials impacts immunogenicity risks, ethical considerations, and scalability for clinical applications in regenerative medicine.
Immunological Challenges and Solutions
Xenotransplantation faces significant immunological challenges, including hyperacute rejection caused by pre-formed natural antibodies targeting alpha-gal epitopes on porcine tissues, necessitating genetic modifications like GGTA1 knockout pigs to mitigate immune response. In vitro organogenesis allows for autologous tissue generation using patient-derived iPSCs, minimizing immune rejection but struggles with replicating complex vascularization and full organ functionality outside the body. Both approaches require advanced immunomodulation strategies, such as CRISPR-mediated gene editing or bioengineered tolerance induction, to overcome host immune system barriers and improve graft survival rates.
Ethical and Regulatory Considerations
Xenotransplantation raises significant ethical concerns regarding animal welfare, cross-species disease transmission, and informed consent, prompting stringent regulatory oversight by agencies such as the FDA and EMA. In vitro organogenesis offers a potentially less controversial alternative by generating human-compatible tissues using stem cells, reducing reliance on animal models and associated bioethical issues. Regulatory frameworks for in vitro organogenesis emphasize ensuring the safety, efficacy, and ethical use of human-derived cells while addressing challenges in scalability and clinical translation.
Current Clinical Applications and Trials
Xenotransplantation involves transplanting organs or tissues from genetically modified animals, primarily pigs, into humans, with clinical trials targeting heart and kidney transplants demonstrating preliminary success in reducing rejection through CRISPR gene editing. In vitro organogenesis focuses on growing human organs from stem cells in bioreactors, currently in experimental stages with ongoing trials exploring lab-grown liver and kidney tissues for transplantation and drug testing. Both fields aim to address organ shortages, with xenotransplantation advancing faster in clinical application, while in vitro organogenesis offers long-term potential for personalized, immunologically compatible organs.
Future Prospects in Organ Replacement Therapy
Xenotransplantation offers the potential to alleviate organ shortages by using genetically engineered animal organs, yet challenges in immune rejection and cross-species disease transmission remain critical hurdles. In vitro organogenesis leverages stem cell technology to create patient-specific organs, promising reduced immunogenicity and personalized treatment options. Advances in gene editing, biomaterials, and 3D bioprinting are accelerating progress in both fields, positioning them as complementary approaches for future organ replacement therapies.
Comparative Analysis: Benefits and Limitations
Xenotransplantation offers the advantage of immediate organ availability from genetically modified animal donors, reducing transplant waiting times but carries risks of immune rejection and cross-species disease transmission. In vitro organogenesis enables personalized organ development using patient-derived stem cells, minimizing immune compatibility issues, yet faces significant challenges in replicating complex tissue architecture and ensuring functional integration. Both approaches are pivotal in addressing organ shortages but require advancements in immunology, bioengineering, and regulatory frameworks to realize clinical viability.
Immunosuppression
Xenotransplantation requires lifelong immunosuppression to prevent organ rejection, whereas in vitro organogenesis offers potential for patient-specific tissues with reduced immunosuppressive needs.
Chimerism
Chimerism in xenotransplantation involves the integration of donor animal cells into human hosts, whereas in vitro organogenesis achieves chimerism by cultivating hybrid tissues combining human and animal stem cells to generate functional organs.
Scaffold biocompatibility
Scaffold biocompatibility in xenotransplantation faces immune rejection challenges, whereas in vitro organogenesis utilizes compatible biomaterials to enhance cell integration and functional tissue development.
Host-graft rejection
Xenotransplantation faces significant challenges with host-graft rejection due to immune incompatibility between species, whereas in vitro organogenesis offers reduced rejection risk by using patient-specific stem cells to grow compatible organs.
Decellularization
Decellularization, a key technique in in vitro organogenesis, involves removing cellular components from donor organs to create scaffolds for tissue regeneration, contrasting with xenotransplantation's use of whole animal organs for transplantation.
Vascularization
Xenotransplantation faces immune rejection challenges in vascularization of transplanted organs, whereas in vitro organogenesis enables controlled vascular network development to improve graft integration and function.
Zoonoses
Xenotransplantation poses a higher risk of zoonoses transmission compared to in vitro organogenesis due to direct cross-species tissue integration and potential viral reservoirs in donor animals.
Pluripotent stem cells
Pluripotent stem cells play a crucial role in xenotransplantation by enabling the generation of immunocompatible tissues, while in vitro organogenesis harnesses these cells to bioengineer fully functional organs for transplantation.
Genetic modification
Genetic modification in xenotransplantation involves editing donor animal genomes to reduce immune rejection, whereas in vitro organogenesis relies on gene editing of human stem cells to grow compatible organs.
Tissue engineering
Tissue engineering in xenotransplantation typically involves modifying animal organs to reduce immunogenicity for transplantation, whereas in vitro organogenesis focuses on growing fully functional human tissues and organs from stem cells to eliminate rejection risks.
Xenotransplantation vs In vitro organogenesis Infographic
 njnir.com
njnir.com