Plasmids are small, circular DNA molecules used as cloning vectors due to their ability to replicate independently within bacterial cells, making them ideal for gene cloning and expression. Cosmids combine features of plasmids and bacteriophage lambda, allowing them to carry larger DNA fragments, typically up to 45 kb, which facilitates the cloning of bigger genetic sequences. The choice between plasmids and cosmids depends on the size of the DNA insert and the specific requirements of the genetic engineering experiment.
Table of Comparison
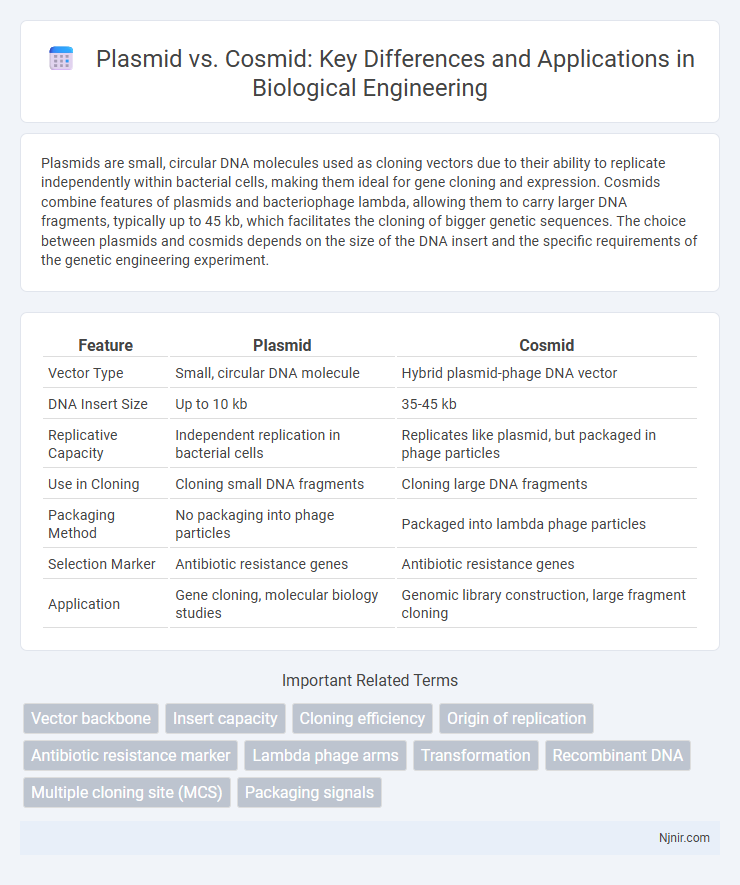
| Feature | Plasmid | Cosmid |
|---|---|---|
| Vector Type | Small, circular DNA molecule | Hybrid plasmid-phage DNA vector |
| DNA Insert Size | Up to 10 kb | 35-45 kb |
| Replicative Capacity | Independent replication in bacterial cells | Replicates like plasmid, but packaged in phage particles |
| Use in Cloning | Cloning small DNA fragments | Cloning large DNA fragments |
| Packaging Method | No packaging into phage particles | Packaged into lambda phage particles |
| Selection Marker | Antibiotic resistance genes | Antibiotic resistance genes |
| Application | Gene cloning, molecular biology studies | Genomic library construction, large fragment cloning |
Introduction to Plasmids and Cosmids
Plasmids are small, circular, double-stranded DNA molecules found in bacteria, used as vectors in genetic engineering due to their ability to replicate independently within host cells. Cosmids are hybrid vectors combining features of plasmids and bacteriophage l, carrying larger DNA inserts up to 45 kb, facilitating efficient DNA cloning and packaging into phage particles. Both plasmids and cosmids serve crucial roles in molecular cloning, with plasmids preferred for smaller gene inserts and cosmids for larger genomic fragments.
Structural Differences Between Plasmids and Cosmids
Plasmids are circular, double-stranded DNA molecules typically ranging from 1 to 100 kilobase pairs that exist independently of chromosomal DNA in bacteria. Cosmids combine features of plasmids and bacteriophage l, containing cos sites that allow packaging into phage particles, and they generally have larger cloning capacities between 35 to 45 kilobase pairs. The structural difference lies in the presence of cos sequences in cosmids enabling efficient DNA packaging and transfer, whereas plasmids lack such sequences and rely solely on replication within the host cell.
Origins and Discovery of Plasmids and Cosmids
Plasmids were first discovered in the 1950s as small, circular DNA molecules capable of independent replication within bacterial cells, originating from studies on bacterial genetics and antibiotic resistance. Cosmids emerged in the 1980s as hybrid cloning vectors combining plasmid origins of replication with cos sites derived from bacteriophage lambda, allowing packaging of larger DNA fragments for gene cloning. The unique replication origins of plasmids enable autonomous replication, while cosmids utilize lambda phage packaging mechanisms to facilitate the cloning of DNA fragments up to 45 kb.
Mechanism of DNA Replication
Plasmids replicate independently of the bacterial chromosome using a theta replication mechanism initiated at a specific origin of replication (ori), involving bidirectional synthesis that produces two circular DNA molecules. Cosmids, which combine features of plasmids and phage l DNA, replicate as plasmids using a similar theta replication process but can carry larger DNA fragments due to their cos sites enabling packaging into phage particles. The replication efficiency of plasmids and cosmids is influenced by the origin of replication type, copy number control, and host strain compatibility, impacting their utility in cloning and genetic manipulation experiments.
Cloning Capacity: Plasmids vs Cosmids
Plasmids typically have a cloning capacity of up to 10 kilobases, making them suitable for cloning smaller DNA fragments. Cosmids can accommodate larger DNA inserts, ranging from 35 to 45 kilobases, combining features of plasmids and bacteriophage l, which allows for efficient packaging and transfer. This higher cloning capacity of cosmids makes them ideal for genomic library construction and large DNA fragment cloning compared to plasmids.
Applications in Genetic Engineering
Plasmids are widely used as vectors for gene cloning and recombinant protein production due to their high copy number and ease of manipulation in bacterial cells. Cosmids combine features of plasmids and bacteriophage l, enabling the cloning of larger DNA fragments (up to 45 kb), which is essential for constructing genomic libraries and mapping complex genomes. Both plasmids and cosmids serve as crucial tools in genetic engineering for gene transfer, functional analysis, and the development of genetically modified organisms.
Advantages and Limitations of Plasmids
Plasmids offer advantages such as ease of manipulation, high copy number, and efficient replication in bacterial hosts, making them ideal for cloning small DNA fragments up to 15 kb. Their limitations include a restricted insert size capacity compared to cosmids, which can carry larger DNA fragments (up to 45 kb) and are suitable for genomic library construction. Plasmids may exhibit instability with large inserts and sometimes require selective markers to maintain plasmid presence in host cells.
Advantages and Limitations of Cosmids
Cosmids offer advantages such as the ability to carry larger DNA fragments (up to 45 kb) compared to plasmids, facilitating the cloning of bigger genetic sequences which is essential in genomic library construction. Their hybrid nature, combining features of plasmids and phage l, allows efficient packaging into phage particles, increasing transformation efficiency and enabling easier transfer of large DNA inserts into host cells. However, cosmids have limitations including a more complex preparation process than plasmids, potential instability of large inserts during replication, and the need for specialized packaging systems, making them less convenient for routine cloning tasks.
Selection Markers and Screening Methods
Plasmids commonly utilize antibiotic resistance genes such as ampicillin or kanamycin for selection markers, enabling the identification of successfully transformed cells through growth on selective media. Cosmids also employ antibiotic resistance markers but often require additional screening methods like blue-white screening due to their capacity to carry larger DNA fragments, facilitating the detection of recombinant clones. Screening approaches for plasmids typically involve colony PCR or restriction digestion, while cosmids benefit from more complex assays including hybridization or size-based screening to confirm insert integration.
Future Perspectives in Vector Design
Future perspectives in vector design highlight the increasing integration of CRISPR-Cas systems with plasmid and cosmid vectors to enhance gene editing precision and efficiency. Advances in synthetic biology are driving the development of modular plasmid and cosmid vectors with expanded cloning capacities and improved stability for complex genome engineering applications. Emerging trends also emphasize the creation of hybrid vectors combining the high-copy number advantages of plasmids with the large DNA insert capacity of cosmids to meet the demands of next-generation therapeutic and industrial biotechnology.
Vector backbone
Plasmid vectors feature a simple circular DNA backbone ideal for cloning small DNA fragments up to 10 kb, while cosmid vectors combine features of plasmids and bacteriophage l, possessing a larger backbone that accommodates DNA inserts between 35-45 kb.
Insert capacity
Cosmids typically have a larger insert capacity of 35-45 kb compared to plasmids, which generally accommodate inserts of up to 10 kb.
Cloning efficiency
Cosmids exhibit higher cloning efficiency than plasmids due to their ability to accommodate larger DNA fragments, typically up to 45 kb compared to plasmids' 10 kb limit.
Origin of replication
Plasmids typically contain a single origin of replication allowing autonomous replication in bacteria, whereas cosmids combine plasmid origins with cos sites enabling packaging into phage particles for efficient DNA cloning.
Antibiotic resistance marker
Plasmids and cosmids both utilize antibiotic resistance markers for selection, but plasmids typically carry a single antibiotic resistance gene, while cosmids often incorporate multiple or more versatile resistance markers to facilitate cloning of larger DNA fragments.
Lambda phage arms
Cosmids combine plasmid replication with lambda phage cos sites enabling efficient packaging and infection of large DNA fragments, unlike plasmids which lack lambda phage arms and have limited cloning capacity.
Transformation
Cosmids typically enable higher transformation efficiency than plasmids due to their larger DNA capacity and ability to incorporate larger genetic fragments during cloning.
Recombinant DNA
Plasmids and cosmids are key recombinant DNA vectors where plasmids are small, circular DNA molecules for cloning short DNA fragments, while cosmids combine plasmid and phage l features enabling cloning of larger DNA segments up to 45 kb.
Multiple cloning site (MCS)
Cosmids typically contain a larger multiple cloning site (MCS) than plasmids, enabling the insertion of more extensive DNA fragments during cloning.
Packaging signals
Plasmids lack specific packaging signals required for viral capsid encapsulation, whereas cosmids contain cohesive end sequences (cos sites) that enable efficient packaging into bacteriophage l particles for transduction.
Plasmid vs Cosmid Infographic
 njnir.com
njnir.com