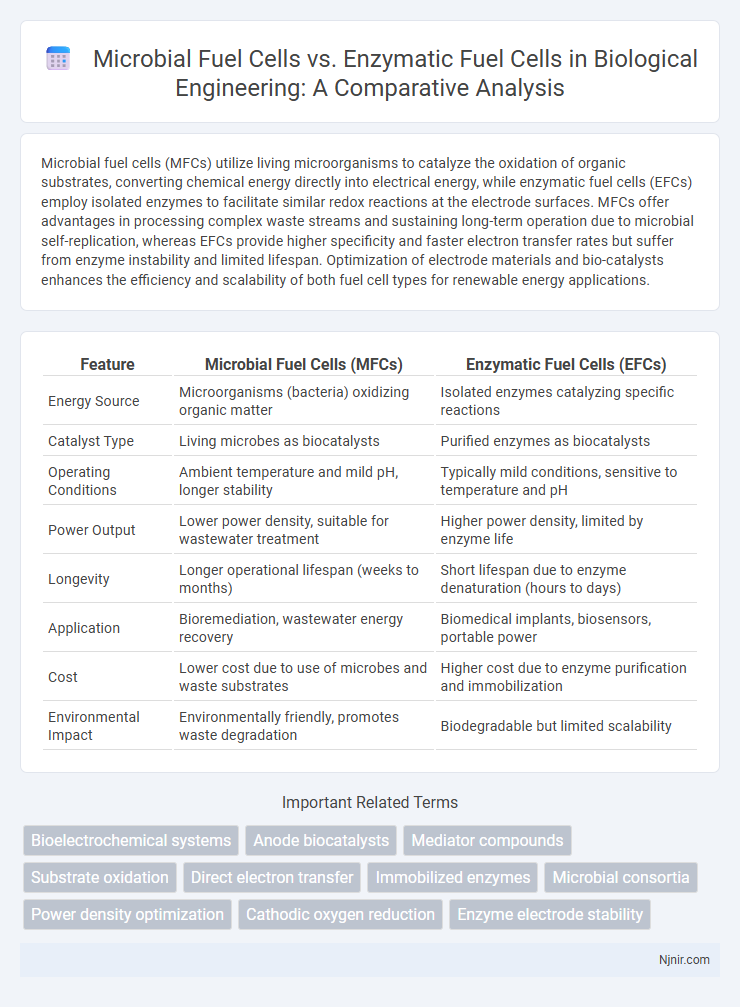
Microbial fuel cells (MFCs) utilize living microorganisms to catalyze the oxidation of organic substrates, converting chemical energy directly into electrical energy, while enzymatic fuel cells (EFCs) employ isolated enzymes to facilitate similar redox reactions at the electrode surfaces. MFCs offer advantages in processing complex waste streams and sustaining long-term operation due to microbial self-replication, whereas EFCs provide higher specificity and faster electron transfer rates but suffer from enzyme instability and limited lifespan. Optimization of electrode materials and bio-catalysts enhances the efficiency and scalability of both fuel cell types for renewable energy applications.
Table of Comparison
| Feature | Microbial Fuel Cells (MFCs) | Enzymatic Fuel Cells (EFCs) |
|---|---|---|
| Energy Source | Microorganisms (bacteria) oxidizing organic matter | Isolated enzymes catalyzing specific reactions |
| Catalyst Type | Living microbes as biocatalysts | Purified enzymes as biocatalysts |
| Operating Conditions | Ambient temperature and mild pH, longer stability | Typically mild conditions, sensitive to temperature and pH |
| Power Output | Lower power density, suitable for wastewater treatment | Higher power density, limited by enzyme life |
| Longevity | Longer operational lifespan (weeks to months) | Short lifespan due to enzyme denaturation (hours to days) |
| Application | Bioremediation, wastewater energy recovery | Biomedical implants, biosensors, portable power |
| Cost | Lower cost due to use of microbes and waste substrates | Higher cost due to enzyme purification and immobilization |
| Environmental Impact | Environmentally friendly, promotes waste degradation | Biodegradable but limited scalability |
Overview of Bioelectrochemical Energy Systems
Microbial fuel cells (MFCs) utilize living microorganisms to catalyze the oxidation of organic substrates, generating electricity through electron transfer processes in bioelectrochemical energy systems. Enzymatic fuel cells (EFCs) employ isolated enzymes as biocatalysts to facilitate specific redox reactions, offering higher specificity and faster reaction rates compared to MFCs. Both technologies represent innovative bioelectrochemical energy systems harnessing biological catalysts for sustainable energy conversion, with MFCs providing broader substrate versatility and EFCs excelling in targeted applications.
Fundamental Principles of Microbial Fuel Cells
Microbial fuel cells (MFCs) generate electricity by harnessing the metabolic activity of electroactive bacteria that oxidize organic substrates and transfer electrons directly to the anode through extracellular electron transfer mechanisms. Unlike enzymatic fuel cells, which rely on isolated enzymes to catalyze reactions, MFCs utilize whole microbial communities, enabling continuous electron flow and biofilm formation on electrode surfaces. The fundamental principle involves converting chemical energy from organic matter into electrical energy via bioelectrochemical processes driven by microbial respiration.
Core Mechanisms of Enzymatic Fuel Cells
Enzymatic fuel cells (EFCs) utilize biocatalysts, primarily enzymes, to convert chemical energy from substrates such as glucose or lactate directly into electrical energy through redox reactions at the bioanode and biocathode. The core mechanism involves specific enzymes like glucose oxidase or laccase facilitating electron transfer by catalyzing substrate oxidation and reduction processes, enabling efficient electron flow within the cell. Compared to microbial fuel cells, which harness whole microorganisms for electron transfer via complex metabolic pathways and extracellular electron transport, EFCs rely on isolated enzymes for higher specificity and faster electron kinetics but often face challenges like enzyme stability and limited operational lifetimes.
Comparative Electrode Materials and Design
Microbial fuel cells (MFCs) commonly utilize carbon-based electrodes like graphite and carbon cloth, chosen for their biocompatibility and high surface area to support biofilm growth, whereas enzymatic fuel cells (EFCs) often incorporate conductive polymers and nanomaterials such as carbon nanotubes or gold nanoparticles to enhance enzyme immobilization and electron transfer. Electrodes in MFCs are typically designed with porous structures to maximize microbial colonization and substrate diffusion, while EFCs feature immobilization matrices and redox mediators integrated within the electrode to facilitate efficient catalytic activity. The design of MFC electrodes prioritizes durability and long-term operation in complex media, contrasting with EFC electrodes which focus on maximizing enzyme stability and electron transfer rates under controlled conditions.
Electron Transfer Pathways: Microbial vs. Enzymatic Approaches
Microbial fuel cells (MFCs) utilize whole microorganisms as biocatalysts, facilitating electron transfer through complex pathways involving outer membrane cytochromes, conductive pili, and redox mediators, enabling efficient extracellular electron transport. Enzymatic fuel cells (EFCs), by contrast, rely on isolated enzymes that directly transfer electrons at the electrode interface, often enhanced by redox-active mediators or conductive nanomaterials to overcome low electron transfer rates and enzyme immobilization challenges. The microbial approach offers robust biofilm formation and versatile substrate utilization, while enzymatic methods provide higher specificity and faster catalytic turnover but face stability and electron transfer limitations.
Power Output and Efficiency Differences
Microbial fuel cells (MFCs) typically generate power outputs ranging from microwatts to milliwatts per square centimeter, leveraging whole microorganisms to oxidize organic substrates, whereas enzymatic fuel cells (EFCs) achieve higher power densities by directly utilizing specific enzymes for catalysis. MFCs often exhibit lower efficiency due to complex electron transfer pathways and biofilm resistance, while EFCs demonstrate improved catalytic efficiency and faster electron transfer rates but face stability challenges limiting long-term performance. The efficiency of EFCs can reach up to 50% under optimized conditions, surpassing the generally lower and variable efficiency of MFCs, which depends heavily on microbial activity and environmental factors.
Substrate Utilization and Fuel Flexibility
Microbial fuel cells (MFCs) utilize a wide range of organic substrates including wastewater, glucose, and complex biomass, offering high fuel flexibility due to diverse microbial communities. Enzymatic fuel cells (EFCs) rely on specific enzymes to catalyze reactions, typically targeting simple substrates like glucose or lactate, resulting in narrower substrate utilization. The adaptability of MFCs to process varied substrates contrasts with the high specificity but limited fuel scope characteristic of EFCs.
Scalability and Practical Applications
Microbial fuel cells (MFCs) demonstrate greater scalability due to their ability to utilize a wide range of organic substrates and operate in diverse environmental conditions, making them suitable for wastewater treatment and remote power generation. Enzymatic fuel cells (EFCs), while offering higher specificity and faster electron transfer rates, face challenges in scalability caused by enzyme instability and limited operational lifespan, restricting their practical applications to biosensors and small-scale portable devices. The scalability advantage of MFCs positions them as promising candidates for large-scale renewable energy production, whereas EFCs excel in precision-demanding fields like medical diagnostics and environmental monitoring.
Environmental Impact and Biocompatibility
Microbial fuel cells (MFCs) utilize living microorganisms to catalyze the conversion of organic matter into electricity, resulting in low environmental impact through waste treatment and renewable energy generation. Enzymatic fuel cells (EFCs) employ isolated enzymes as biocatalysts, offering high specificity and reduced toxicity, thus enhancing biocompatibility in medical and environmental applications. MFCs demonstrate robust biodegradability and ecosystem integration, while EFCs excel in controlled environments requiring minimal immune response and precise biochemical interactions.
Future Prospects and Integration Challenges
Microbial fuel cells (MFCs) exhibit promising potential for sustainable energy generation through the direct conversion of organic waste into electricity, with future advancements targeting enhanced electron transfer and biofilm engineering. Enzymatic fuel cells (EFCs) offer high specificity and efficiency in catalyzing biochemical reactions, yet their widespread integration faces challenges related to enzyme stability, cost, and limited operational lifespan. Overcoming electrode material limitations and improving system scalability remain critical hurdles for both MFCs and EFCs to achieve commercial viability in renewable energy applications.
Bioelectrochemical systems
Microbial fuel cells leverage electroactive microorganisms to generate electricity from organic substrates, whereas enzymatic fuel cells use isolated enzymes for catalysis, both representing bioelectrochemical systems that convert biochemical energy into electrical energy through distinct biocatalytic mechanisms.
Anode biocatalysts
Microbial fuel cells utilize whole microorganisms as anode biocatalysts for sustainable electron transfer, while enzymatic fuel cells rely on isolated enzymes offering higher specificity and faster catalytic rates for efficient energy conversion.
Mediator compounds
Microbial fuel cells utilize naturally produced or synthetic mediators like quinones to facilitate electron transfer from microbes to electrodes, whereas enzymatic fuel cells often rely on redox mediators such as osmium complexes to shuttle electrons between enzymes and electrodes for enhanced efficiency.
Substrate oxidation
Microbial fuel cells harness diverse microorganisms to oxidize complex organic substrates, whereas enzymatic fuel cells rely on specific enzymes to catalyze the oxidation of targeted substrates for efficient electron transfer.
Direct electron transfer
Microbial fuel cells enable direct electron transfer through biofilm-forming bacteria on electrodes, while enzymatic fuel cells rely on enzyme-catalyzed reactions with redox mediators to facilitate electron transfer.
Immobilized enzymes
Immobilized enzymes in enzymatic fuel cells enhance electron transfer efficiency and operational stability compared to microbial fuel cells, which rely on whole-cell metabolism for bioelectricity generation.
Microbial consortia
Microbial fuel cells leverage diverse microbial consortia to enhance electron transfer efficiency and substrate versatility compared to the more substrate-specific enzymatic fuel cells.
Power density optimization
Microbial fuel cells achieve power density optimization through biofilm thickness and electrode material enhancements, while enzymatic fuel cells optimize power density primarily by enzyme immobilization techniques and substrate concentration control.
Cathodic oxygen reduction
Microbial fuel cells utilize biofilms of microorganisms for cathodic oxygen reduction, offering greater durability and scalability, whereas enzymatic fuel cells rely on specific enzymes like laccase or bilirubin oxidase for higher catalytic efficiency but lower operational stability.
Enzyme electrode stability
Enzymatic fuel cells face significant challenges with enzyme electrode stability due to rapid enzyme degradation and limited operational lifespan compared to the more robust microbial fuel cells.
Microbial fuel cells vs Enzymatic fuel cells Infographic
 njnir.com
njnir.com