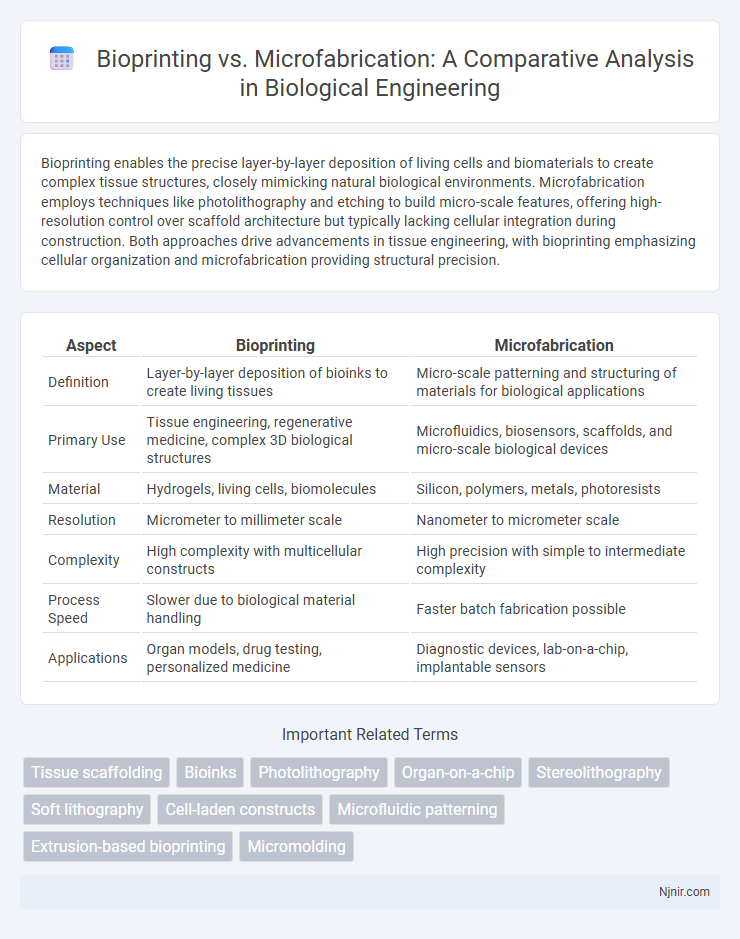
Bioprinting enables the precise layer-by-layer deposition of living cells and biomaterials to create complex tissue structures, closely mimicking natural biological environments. Microfabrication employs techniques like photolithography and etching to build micro-scale features, offering high-resolution control over scaffold architecture but typically lacking cellular integration during construction. Both approaches drive advancements in tissue engineering, with bioprinting emphasizing cellular organization and microfabrication providing structural precision.
Table of Comparison
| Aspect | Bioprinting | Microfabrication |
|---|---|---|
| Definition | Layer-by-layer deposition of bioinks to create living tissues | Micro-scale patterning and structuring of materials for biological applications |
| Primary Use | Tissue engineering, regenerative medicine, complex 3D biological structures | Microfluidics, biosensors, scaffolds, and micro-scale biological devices |
| Material | Hydrogels, living cells, biomolecules | Silicon, polymers, metals, photoresists |
| Resolution | Micrometer to millimeter scale | Nanometer to micrometer scale |
| Complexity | High complexity with multicellular constructs | High precision with simple to intermediate complexity |
| Process Speed | Slower due to biological material handling | Faster batch fabrication possible |
| Applications | Organ models, drug testing, personalized medicine | Diagnostic devices, lab-on-a-chip, implantable sensors |
Introduction to Bioprinting and Microfabrication
Bioprinting utilizes 3D printing technology to fabricate tissue-like structures by precisely depositing bioinks containing living cells, enabling the creation of complex biological architectures for regenerative medicine. Microfabrication involves the manipulation of materials at the microscale using techniques such as photolithography and etching to produce microdevices and scaffolds for biomedical applications. Both approaches contribute to tissue engineering by offering distinct capabilities in constructing functional biological systems with high spatial resolution.
Fundamental Principles of Bioprinting
Bioprinting relies on layer-by-layer deposition of bioinks containing living cells and biomaterials to create 3D tissue constructs, while microfabrication employs top-down lithographic techniques to pattern microstructures primarily from non-living substrates. The fundamental principles of bioprinting include precise spatial control of cell placement, maintenance of cell viability during printing, and the use of bioinks that support cell function and mimic native extracellular matrices. These principles enable bioprinting to fabricate complex, functional tissues with biological, mechanical, and structural fidelity that microfabrication alone cannot achieve.
Core Techniques in Microfabrication
Microfabrication primarily involves photolithography, etching, and deposition techniques to create intricate micro-scale structures essential for biomedical devices. Photolithography uses light to pattern substrates with precision, while etching selectively removes material to form microchannels and features. Deposition techniques like chemical vapor deposition (CVD) and sputtering allow for controlled layering of materials, enabling the fabrication of complex microarchitectures critical in tissue engineering and organ-on-a-chip platforms.
Materials Used in Both Technologies
Bioprinting primarily utilizes bioinks composed of living cells, hydrogels, and growth factors, enabling the creation of complex tissue constructs with high biocompatibility and functionality. Microfabrication relies on materials such as silicon, polymers like PDMS, and photolithographic resists, providing precise control over micro-scale features for applications in microfluidics and lab-on-a-chip devices. The selection of materials in bioprinting prioritizes biological compatibility and cellular viability, while microfabrication materials emphasize structural integrity and micro-scale precision.
Resolution and Precision: A Comparative Analysis
Bioprinting and microfabrication differ significantly in resolution and precision, with microfabrication achieving nanoscale accuracy often below 100 nanometers, ideal for creating intricate microstructures. Bioprinting typically offers resolutions ranging from 100 micrometers to several hundred micrometers, which suits larger, complex biological constructs but limits fine cellular detail replication. The precision of microfabrication enables highly controlled patterning at the molecular level, whereas bioprinting prioritizes viable tissue formation with variable precision influenced by bioink properties and printing technology.
Applications in Tissue Engineering
Bioprinting enables precise layering of living cells and biomaterials to create complex, three-dimensional tissues, making it ideal for scaffold fabrication in regenerative medicine. Microfabrication techniques provide microscale control over scaffold architecture and microenvironment, crucial for mimicking native tissue structures and enhancing cell behavior. Both methods contribute to developing functional tissue constructs, with bioprinting excelling in versatility and microfabrication offering superior resolution and microstructural fidelity.
Scalability and Throughput Considerations
Bioprinting offers high scalability by enabling the automated deposition of living cells and biomaterials in complex, three-dimensional structures, which facilitates rapid production of tissue constructs. Microfabrication techniques provide precise control at the microscale but often face limitations in throughput due to intricate lithography and etching processes. Balancing bioprinting's scalability with microfabrication's precision is critical for advancing large-scale tissue engineering and personalized medicine applications.
Challenges and Limitations
Bioprinting faces challenges such as limited bioink materials, low resolution compared to native tissue complexity, and difficulties in vascularization for thick tissue constructs. Microfabrication offers high precision and reproducibility but is constrained by material compatibility issues and inability to replicate the dynamic biological environment. Both techniques struggle with scalability and integrating functional cell types to fully mimic native tissue architecture and function.
Future Prospects and Emerging Trends
Future prospects in bioprinting emphasize advancements in tissue engineering with enhanced precision and scalability, enabling the creation of complex, functional organs for transplantation. Microfabrication continues to evolve with innovations like nanoscale patterning and MEMS integration, improving device miniaturization and biomimetic surface designs for cell studies. Emerging trends reveal a convergence of bioprinting and microfabrication technologies, fostering hybrid systems that leverage precise material deposition alongside microstructured environments to accelerate regenerative medicine and personalized healthcare solutions.
Conclusion: Selecting the Optimal Approach
Selecting the optimal approach between bioprinting and microfabrication depends on the specific application requirements such as resolution, material complexity, and biological compatibility. Bioprinting excels in creating complex tissue structures with viable cells, making it ideal for regenerative medicine and tissue engineering. Microfabrication offers superior precision and scalability for creating microfluidic devices and biosensors, favoring applications that require intricate micro-scale designs and non-cellular components.
Tissue scaffolding
Bioprinting enables precise, customizable tissue scaffolding by depositing living cells layer-by-layer, while microfabrication offers high-resolution scaffold microstructures through controlled physical patterning techniques.
Bioinks
Bioinks in bioprinting offer customizable, cell-laden formulations enabling precise tissue construction, whereas microfabrication employs synthetic, scaffold-based materials prioritizing structural microfeatures over biological functionality.
Photolithography
Photolithography in microfabrication enables precise patterning of biocompatible materials on a microscale, whereas bioprinting offers layer-by-layer deposition of living cells and biomaterials for complex tissue constructs.
Organ-on-a-chip
Organ-on-a-chip systems leverage bioprinting for precise 3D cellular architectures, while microfabrication offers high-resolution microchannel patterning essential for simulating organ-level functions.
Stereolithography
Stereolithography in bioprinting offers higher resolution and complex 3D tissue structures compared to traditional microfabrication methods, enhancing precision in regenerative medicine applications.
Soft lithography
Soft lithography enables precise microfabrication of bioprinted tissues by creating flexible, high-resolution polymer molds that guide cell patterning and scaffold design for regenerative medicine applications.
Cell-laden constructs
Cell-laden constructs in bioprinting enable precise spatial arrangement of living cells within 3D scaffolds, whereas microfabrication offers high-resolution patterning but often lacks cell viability during fabrication.
Microfluidic patterning
Microfluidic patterning in microfabrication enables precise manipulation of fluids at the microscale, offering superior spatial resolution and control compared to bioprinting techniques for creating complex tissue architectures.
Extrusion-based bioprinting
Extrusion-based bioprinting offers precise, layer-by-layer fabrication of complex, cell-laden structures, providing superior customization and scalability compared to traditional microfabrication techniques used in tissue engineering.
Micromolding
Micromolding, a key technique in microfabrication, enables precise replication of micro-scale structures essential for bioprinting applications in tissue engineering and regenerative medicine.
Bioprinting vs Microfabrication Infographic
 njnir.com
njnir.com