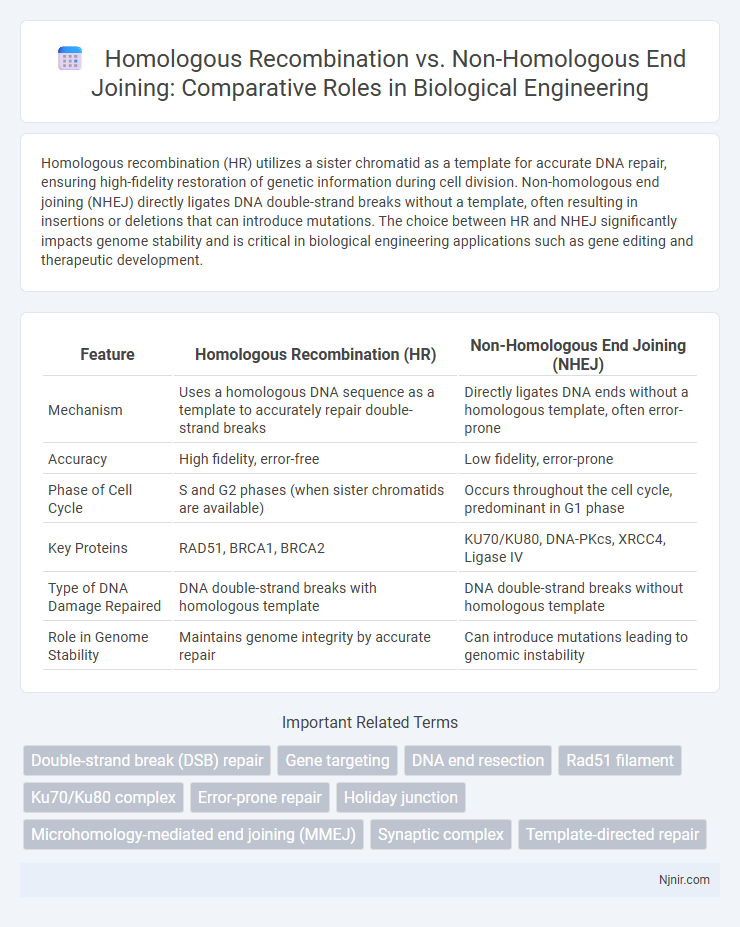
Homologous recombination (HR) utilizes a sister chromatid as a template for accurate DNA repair, ensuring high-fidelity restoration of genetic information during cell division. Non-homologous end joining (NHEJ) directly ligates DNA double-strand breaks without a template, often resulting in insertions or deletions that can introduce mutations. The choice between HR and NHEJ significantly impacts genome stability and is critical in biological engineering applications such as gene editing and therapeutic development.
Table of Comparison
| Feature | Homologous Recombination (HR) | Non-Homologous End Joining (NHEJ) |
|---|---|---|
| Mechanism | Uses a homologous DNA sequence as a template to accurately repair double-strand breaks | Directly ligates DNA ends without a homologous template, often error-prone |
| Accuracy | High fidelity, error-free | Low fidelity, error-prone |
| Phase of Cell Cycle | S and G2 phases (when sister chromatids are available) | Occurs throughout the cell cycle, predominant in G1 phase |
| Key Proteins | RAD51, BRCA1, BRCA2 | KU70/KU80, DNA-PKcs, XRCC4, Ligase IV |
| Type of DNA Damage Repaired | DNA double-strand breaks with homologous template | DNA double-strand breaks without homologous template |
| Role in Genome Stability | Maintains genome integrity by accurate repair | Can introduce mutations leading to genomic instability |
Introduction to DNA Double-Strand Break Repair Mechanisms
DNA double-strand break (DSB) repair mechanisms are critical for maintaining genomic stability, primarily involving homologous recombination (HR) and non-homologous end joining (NHEJ). HR utilizes a homologous DNA sequence as a template for accurate repair, predominantly during the S and G2 phases of the cell cycle, ensuring error-free restoration. NHEJ directly ligates broken DNA ends without a template, functioning throughout the cell cycle but often resulting in small insertions or deletions, making it a faster yet error-prone repair pathway.
Overview of Homologous Recombination
Homologous recombination (HR) is a precise DNA repair mechanism that fixes double-strand breaks using a homologous sequence as a template, typically the sister chromatid, ensuring high-fidelity restoration of genetic information. This process is essential during the S and G2 phases of the cell cycle when sister chromatids are available. Key proteins involved in HR include RAD51, BRCA1, and BRCA2, which facilitate strand invasion and exchange, promoting accurate DNA repair and maintaining genomic stability.
Overview of Non-Homologous End Joining
Non-Homologous End Joining (NHEJ) is a DNA repair mechanism that directly ligates broken DNA ends without requiring a homologous template, making it the predominant pathway for repairing double-strand breaks in mammalian cells. NHEJ involves key proteins such as Ku70/Ku80 heterodimer that recognize DNA ends, DNA-PKcs that facilitates end processing, and the Ligase IV complex responsible for sealing the breaks. Compared to Homologous Recombination, NHEJ is faster but more error-prone, often leading to small insertions or deletions at the repair site.
Molecular Processes Underlying Homologous Recombination
Homologous recombination involves the precise repair of DNA double-strand breaks by using an undamaged homologous sequence as a template, ensuring accurate genetic information restoration. The molecular process initiates with end resection to produce 3' single-stranded DNA overhangs, followed by RAD51-mediated strand invasion and homology search within the sister chromatid or homologous chromosome. DNA synthesis then occurs to extend the invading strand, ultimately leading to resolution of Holliday junctions and restoration of genomic integrity without loss of genetic information.
Key Proteins Involved in Non-Homologous End Joining
Non-Homologous End Joining (NHEJ) primarily involves key proteins such as Ku70/Ku80 heterodimer, DNA-PKcs, XRCC4, Ligase IV, and XLF, which collaborate to recognize, process, and ligate double-strand DNA breaks without a homologous template. Ku70/Ku80 binds to DNA ends, recruiting DNA-PKcs to form the DNA-PK complex that stabilizes the broken ends and facilitates end processing. XRCC4 and Ligase IV form a complex essential for the final ligation step, with XLF enhancing ligation efficiency, collectively ensuring rapid and efficient repair in mammalian cells.
Differences in Accuracy and Fidelity: HR vs NHEJ
Homologous recombination (HR) exhibits high accuracy and fidelity by utilizing a homologous template for error-free DNA double-strand break repair, preserving genomic integrity. Non-homologous end joining (NHEJ) is faster but error-prone, often resulting in insertions or deletions due to direct ligation without a template. HR primarily operates during the S and G2 phases of the cell cycle, while NHEJ functions throughout, highlighting their distinct repair mechanisms and impacts on genomic stability.
Regulation of Repair Pathway Choice in Eukaryotic Cells
Eukaryotic cells regulate DNA double-strand break repair pathway choice through cell cycle-dependent mechanisms, favoring homologous recombination (HR) in the S and G2 phases when a sister chromatid is available as a template. Non-homologous end joining (NHEJ) predominates in the G1 phase, influenced by the activity of key proteins such as 53BP1 and BRCA1, which modulate DNA end resection and pathway commitment. Post-translational modifications and chromatin state also play crucial roles in determining the accessibility and processing of DNA ends, ensuring accurate repair and genomic stability.
Biological Significance in Genome Stability
Homologous recombination (HR) ensures genome stability by accurately repairing DNA double-strand breaks using a homologous template, preserving genetic information and preventing mutations. Non-homologous end joining (NHEJ) quickly repairs breaks without a template, which is crucial for cell survival but can introduce insertions or deletions, potentially compromising genomic integrity. The balance between HR and NHEJ pathways is vital for maintaining chromosomal stability and preventing cancer development.
Applications in Genetic Engineering and Gene Editing
Homologous recombination enables precise gene editing by using a homologous DNA template to guide accurate DNA repair, making it essential for targeted gene insertion or correction in genetic engineering. Non-homologous end joining facilitates rapid DNA double-strand break repair without requiring a template, often introducing insertions or deletions that are useful for gene knockout studies. Both mechanisms are exploited in CRISPR-Cas9 technology, where homologous recombination supports precise gene modifications and non-homologous end joining induces gene disruptions for functional genomics research.
Future Perspectives and Emerging Technologies
Future perspectives in DNA repair emphasize enhancing the precision of homologous recombination (HR) through CRISPR-Cas9 advancements, enabling targeted gene correction with reduced off-target effects. Emerging technologies in non-homologous end joining (NHEJ) involve engineering DNA ligase IV and associated factors to improve efficiency and minimize error-prone repair outcomes. Integration of real-time single-molecule imaging and AI-driven computational models promises to revolutionize understanding and manipulation of both HR and NHEJ pathways for therapeutic applications.
Double-strand break (DSB) repair
Homologous recombination accurately repairs double-strand breaks (DSBs) using a homologous DNA template, while non-homologous end joining directly ligates the broken DNA ends without a template, often resulting in mutations.
Gene targeting
Homologous recombination enables precise gene targeting by using a homologous DNA template for accurate repair, whereas non-homologous end joining facilitates rapid but error-prone repair without a template, leading to insertions or deletions at double-strand breaks.
DNA end resection
Homologous recombination requires extensive DNA end resection to generate 3' single-stranded DNA overhangs for strand invasion, whereas non-homologous end joining involves minimal or no DNA end resection, directly ligating DNA ends to repair double-strand breaks.
Rad51 filament
Rad51 filament plays a crucial role in homologous recombination by facilitating strand invasion and DNA repair accuracy, whereas non-homologous end joining repairs double-strand breaks without Rad51 involvement, resulting in more error-prone outcomes.
Ku70/Ku80 complex
The Ku70/Ku80 complex plays a critical role in non-homologous end joining by recognizing and binding DNA double-strand breaks, whereas homologous recombination relies on RAD51 and BRCA proteins to accurately repair breaks using a homologous template.
Error-prone repair
Non-homologous end joining is an error-prone DNA repair pathway that directly ligates double-strand breaks without a template, while homologous recombination uses a homologous sequence for accurate repair, minimizing mutations.
Holiday junction
Homologous recombination repairs DNA double-strand breaks by forming and resolving a Holliday junction through strand invasion and exchange, while non-homologous end joining directly ligates broken ends without forming such structures.
Microhomology-mediated end joining (MMEJ)
Microhomology-mediated end joining (MMEJ) distinctively repairs DNA double-strand breaks by utilizing short homologous sequences for alignment, contrasting with homologous recombination's extensive sequence homology requirement and non-homologous end joining's direct ligation without homology.
Synaptic complex
The synaptic complex in homologous recombination precisely aligns homologous DNA sequences for accurate repair, whereas in non-homologous end joining, the synaptic complex facilitates direct ligation of DNA ends with minimal sequence homology, often leading to insertions or deletions.
Template-directed repair
Homologous recombination precisely repairs DNA double-strand breaks using a homologous template, whereas non-homologous end joining directly ligates break ends without a template, resulting in higher error rates.
Homologous recombination vs Non-homologous end joining Infographic
 njnir.com
njnir.com