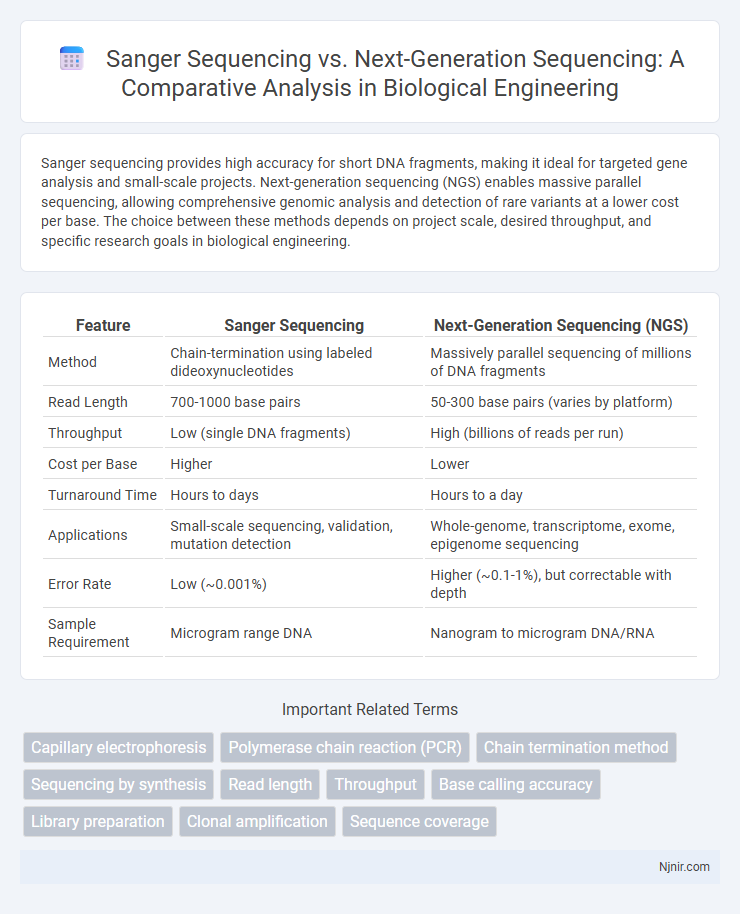
Sanger sequencing provides high accuracy for short DNA fragments, making it ideal for targeted gene analysis and small-scale projects. Next-generation sequencing (NGS) enables massive parallel sequencing, allowing comprehensive genomic analysis and detection of rare variants at a lower cost per base. The choice between these methods depends on project scale, desired throughput, and specific research goals in biological engineering.
Table of Comparison
| Feature | Sanger Sequencing | Next-Generation Sequencing (NGS) |
|---|---|---|
| Method | Chain-termination using labeled dideoxynucleotides | Massively parallel sequencing of millions of DNA fragments |
| Read Length | 700-1000 base pairs | 50-300 base pairs (varies by platform) |
| Throughput | Low (single DNA fragments) | High (billions of reads per run) |
| Cost per Base | Higher | Lower |
| Turnaround Time | Hours to days | Hours to a day |
| Applications | Small-scale sequencing, validation, mutation detection | Whole-genome, transcriptome, exome, epigenome sequencing |
| Error Rate | Low (~0.001%) | Higher (~0.1-1%), but correctable with depth |
| Sample Requirement | Microgram range DNA | Nanogram to microgram DNA/RNA |
Overview of Sanger Sequencing and Next-Generation Sequencing
Sanger sequencing, also known as chain-termination sequencing, provides high-accuracy DNA sequence data by synthesizing DNA fragments labeled with dideoxynucleotides, ideal for sequencing short DNA regions up to 900 base pairs. Next-generation sequencing (NGS) encompasses various high-throughput technologies that parallelize DNA sequencing, producing massively large volumes of data and enabling whole-genome, exome, or transcriptome analysis. While Sanger sequencing remains the gold standard for validating small-scale studies, NGS is favored for large-scale, comprehensive genomic research due to its efficiency and cost-effectiveness.
Historical Background and Technological Evolution
Sanger sequencing, developed by Frederick Sanger in 1977, revolutionized DNA analysis with its chain-termination method, becoming the gold standard for sequencing for over two decades. Next-generation sequencing (NGS) emerged in the mid-2000s, leveraging massively parallel sequencing technologies that dramatically increased throughput and reduced costs. The technological evolution from Sanger's electrophoresis-based platform to NGS's high-throughput, short-read sequencing systems enabled large-scale genomic projects and personalized medicine advancements.
Principles and Workflow of Sanger Sequencing
Sanger sequencing relies on selective incorporation of chain-terminating dideoxynucleotides during DNA synthesis, producing fragments of varying lengths that are separated by capillary electrophoresis to determine the DNA sequence. The workflow involves DNA template preparation, primer annealing, extension with DNA polymerase, incorporation of fluorescently labeled dideoxynucleotides, fragment separation, and automated detection of fluorescent signals. Unlike next-generation sequencing (NGS), which sequences millions of fragments in parallel, Sanger sequencing provides high accuracy with longer read lengths but lower throughput.
Principles and Workflow of Next-Generation Sequencing
Next-generation sequencing (NGS) utilizes massively parallel sequencing technology that reads millions of DNA fragments simultaneously, contrasting with Sanger sequencing's chain termination method which sequences one fragment at a time. The NGS workflow begins with DNA fragmentation, followed by adapter ligation, clonal amplification (such as bridge amplification or emulsion PCR), and high-throughput sequencing by synthesis or ligation. This approach enables rapid, large-scale genomic data generation with higher coverage and sensitivity compared to the low-throughput, longer-read output of Sanger sequencing.
Comparative Analysis: Accuracy and Sensitivity
Sanger sequencing offers high accuracy with an error rate below 0.1%, making it ideal for analyzing single genes or small genomic regions with clear, long-read outputs. Next-generation sequencing (NGS) provides superior sensitivity by detecting low-frequency variants and enabling massive parallel sequencing of whole genomes or exomes, but it typically has a higher raw error rate around 1%. Combining both technologies leverages Sanger's precision for validation and NGS's vast data throughput for comprehensive variant discovery in complex genomic studies.
Throughput and Scalability Differences
Sanger sequencing processes DNA fragments one at a time, resulting in low throughput and limited scalability, making it suitable for small-scale projects. Next-generation sequencing (NGS) simultaneously sequences millions of DNA fragments, offering exponentially higher throughput and scalable solutions for large genomic studies. NGS platforms enable parallel processing and automation, drastically reducing time and cost per base compared to the sequential nature of Sanger sequencing.
Applications in Biological Engineering
Sanger sequencing remains a gold standard for validating small-scale genetic modifications and analyzing specific gene regions in biological engineering. Next-generation sequencing (NGS) enables comprehensive genomic profiling, high-throughput variant detection, and transcriptomic analysis critical for synthetic biology, metabolic engineering, and strain optimization. The scalability and high resolution of NGS accelerate pathway elucidation and precision editing, driving advancements in biofuel production, pharmaceutical development, and microbial engineering.
Cost Analysis and Time Efficiency
Sanger sequencing costs approximately $500 to $1,000 per megabase, making it less cost-effective for large-scale projects, while Next-generation sequencing (NGS) offers reduced costs around $0.01 to $0.10 per megabase due to high-throughput capabilities. In terms of time efficiency, Sanger sequencing can take several days per sample, limiting its use for extensive genomic studies, whereas NGS can process millions of sequences simultaneously within hours to days. The scalability of NGS significantly lowers overall project costs and accelerates turnaround times compared to traditional Sanger methods.
Limitations and Challenges of Each Method
Sanger sequencing offers high accuracy for short DNA fragments but struggles with low throughput and high cost, making it inefficient for whole-genome analysis or large-scale studies. Next-generation sequencing (NGS) provides massive parallelization and scalability but faces challenges such as shorter read lengths, higher error rates, and complex data analysis requirements. Both methods require specialized equipment and expertise, with NGS demanding significant computational resources to manage and interpret vast datasets.
Future Trends in Sequencing Technologies
Future trends in sequencing technologies emphasize increased accuracy, speed, and cost-efficiency, with next-generation sequencing (NGS) platforms advancing towards real-time, portable, and single-molecule sequencing capabilities. Sanger sequencing remains essential for validating NGS data due to its high accuracy but is gradually being supplemented by emerging third-generation technologies such as nanopore and single-molecule real-time (SMRT) sequencing. Integration of artificial intelligence and machine learning algorithms promises enhanced data interpretation and error correction, driving personalized medicine and large-scale genomic studies forward.
Capillary electrophoresis
Sanger sequencing uses capillary electrophoresis to separate DNA fragments by size for high-accuracy single-read sequencing, while next-generation sequencing employs massively parallel sequencing techniques without relying on capillary electrophoresis for faster, high-throughput data generation.
Polymerase chain reaction (PCR)
Next-generation sequencing uses PCR amplification to generate massive parallel DNA libraries enabling high-throughput analysis, whereas Sanger sequencing relies on targeted PCR to amplify individual DNA fragments for precise, low-throughput sequencing.
Chain termination method
Sanger sequencing utilizes the chain termination method by incorporating labeled dideoxynucleotides to terminate DNA synthesis at specific bases, allowing precise sequence determination, whereas next-generation sequencing employs massively parallel sequencing techniques without relying on chain termination.
Sequencing by synthesis
Next-generation sequencing revolutionizes Sequencing by Synthesis with massively parallel processing, delivering higher throughput, speed, and cost-efficiency compared to the traditional Sanger sequencing method.
Read length
Sanger sequencing offers longer read lengths up to 900 base pairs, whereas next-generation sequencing typically produces shorter reads ranging from 50 to 300 base pairs.
Throughput
Next-generation sequencing offers exponentially higher throughput than Sanger sequencing by simultaneously processing millions of DNA fragments, enabling rapid and large-scale genomic analysis.
Base calling accuracy
Sanger sequencing provides higher base calling accuracy with error rates below 0.001%, whereas next-generation sequencing typically has higher error rates ranging from 0.1% to 1% due to its massive parallel processing.
Library preparation
Next-generation sequencing requires more complex library preparation involving DNA fragmentation, adapter ligation, and amplification, whereas Sanger sequencing typically involves simpler, single-fragment template preparation without extensive adapter ligation.
Clonal amplification
Next-generation sequencing relies on clonal amplification of DNA fragments on solid supports, enabling massively parallel sequencing, while Sanger sequencing sequences single DNA molecules without clonal amplification.
Sequence coverage
Next-generation sequencing provides significantly higher sequence coverage than Sanger sequencing, enabling more comprehensive genome analysis and variant detection.
Sanger sequencing vs Next-generation sequencing Infographic
 njnir.com
njnir.com