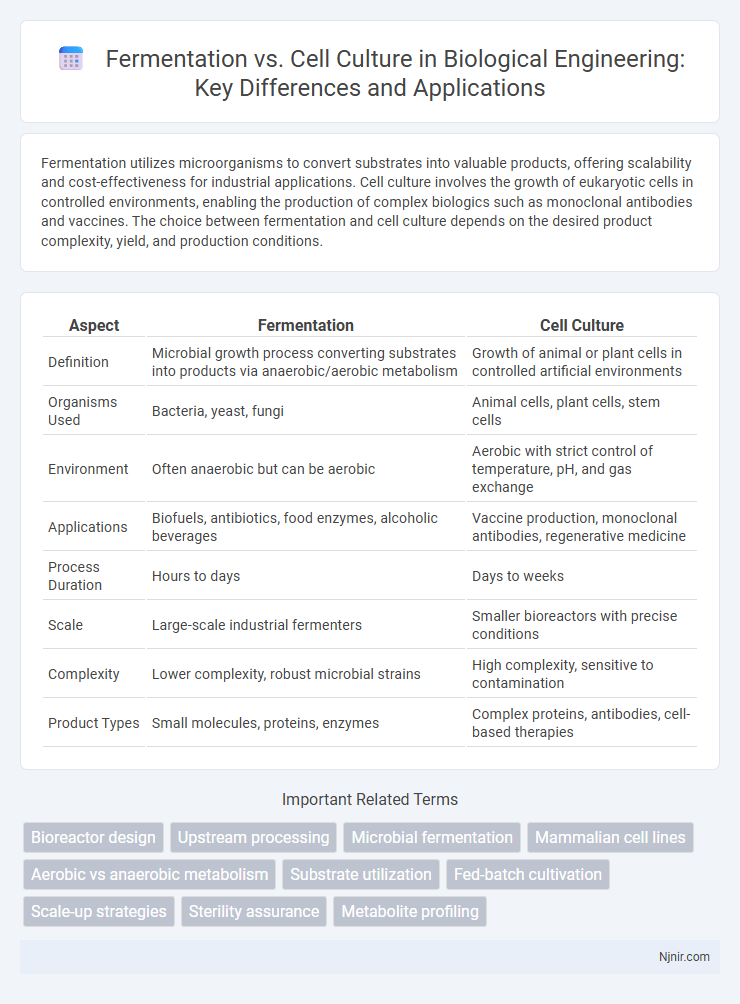
Fermentation utilizes microorganisms to convert substrates into valuable products, offering scalability and cost-effectiveness for industrial applications. Cell culture involves the growth of eukaryotic cells in controlled environments, enabling the production of complex biologics such as monoclonal antibodies and vaccines. The choice between fermentation and cell culture depends on the desired product complexity, yield, and production conditions.
Table of Comparison
| Aspect | Fermentation | Cell Culture |
|---|---|---|
| Definition | Microbial growth process converting substrates into products via anaerobic/aerobic metabolism | Growth of animal or plant cells in controlled artificial environments |
| Organisms Used | Bacteria, yeast, fungi | Animal cells, plant cells, stem cells |
| Environment | Often anaerobic but can be aerobic | Aerobic with strict control of temperature, pH, and gas exchange |
| Applications | Biofuels, antibiotics, food enzymes, alcoholic beverages | Vaccine production, monoclonal antibodies, regenerative medicine |
| Process Duration | Hours to days | Days to weeks |
| Scale | Large-scale industrial fermenters | Smaller bioreactors with precise conditions |
| Complexity | Lower complexity, robust microbial strains | High complexity, sensitive to contamination |
| Product Types | Small molecules, proteins, enzymes | Complex proteins, antibodies, cell-based therapies |
Introduction to Fermentation and Cell Culture
Fermentation is a biochemical process that involves the conversion of organic substrates by microorganisms such as bacteria, yeast, or fungi to produce valuable products like ethanol, antibiotics, or enzymes under anaerobic or aerobic conditions. Cell culture refers to the cultivation of animal, plant, or microbial cells in controlled environments, facilitating research, vaccine production, and biopharmaceutical manufacturing. Both techniques are foundational in biotechnology, enabling large-scale production and experimentation with living cells and biomolecules.
Principles of Fermentation Processes
Fermentation processes rely on anaerobic or aerobic microbial metabolism to convert substrates like sugars into desired products such as ethanol, organic acids, or antibiotics, utilizing microorganisms such as bacteria, yeast, or fungi. Key parameters include temperature, pH, oxygen concentration, and substrate availability, all of which influence microbial growth kinetics and product yield during batch, fed-batch, or continuous fermentation modes. Unlike cell culture, which often involves mammalian or insect cells cultivating proteins in sterile, controlled environments, fermentation emphasizes microbial biomass proliferation and metabolite production through enzymatic pathways optimized by bioreactor design and process engineering.
Fundamentals of Cell Culture Techniques
Cell culture techniques involve growing cells under controlled conditions, typically in a nutrient-rich medium, allowing for the study of cellular behavior, genetic expression, and biochemical processes. Fermentation is a metabolic process harnessed in bioreactors, converting substrates into desired products via microbial or cell cultures, often under anaerobic conditions. Mastery of aseptic technique, optimal media formulation, and environmental control such as pH, temperature, and oxygen levels is fundamental to successful cell culture and fermentation processes.
Key Differences: Fermentation vs. Cell Culture
Fermentation primarily uses microorganisms such as bacteria, yeast, or fungi to convert substrates into desired products under anaerobic or aerobic conditions, while cell culture involves the growth of mammalian, insect, or plant cells in controlled, sterile environments typically for biopharmaceutical production. Fermentation processes often yield metabolites like alcohol, organic acids, or antibiotics through microbial metabolism, whereas cell culture facilitates the production of complex proteins, vaccines, or monoclonal antibodies via eukaryotic cellular machinery. Scale, oxygen requirements, and product complexity are critical differentiators, with fermentation favoring large-scale, simpler molecule output, and cell culture supporting smaller-scale, high-value biologics manufacturing.
Microbial Fermentation: Organisms and Applications
Microbial fermentation utilizes bacteria, yeasts, and molds to convert substrates into valuable products such as antibiotics, enzymes, and biofuels. Common organisms include Saccharomyces cerevisiae for ethanol production and Lactobacillus species for lactic acid fermentation. This process is widely applied in pharmaceuticals, food industries, and renewable energy sectors due to its efficiency and scalability.
Mammalian and Plant Cell Culture Systems
Mammalian and plant cell culture systems serve distinct roles in bioproduction, with mammalian cell cultures primarily used for producing complex therapeutic proteins due to their ability to perform human-like post-translational modifications. Fermentation typically involves microbial systems that offer high yield and rapid growth but lack the sophisticated protein processing found in mammalian cultures. Plant cell culture systems provide an alternative platform with lower contamination risk and scalable production, suitable for vaccine development and bioactive compounds, yet they often require optimization to match mammalian cell culture protein expression levels.
Product Types: Metabolites vs. Biologics
Fermentation primarily produces metabolites such as alcohols, organic acids, and antibiotics through microbial activity, emphasizing small molecule synthesis. Cell culture techniques generate biologics like monoclonal antibodies, vaccines, and recombinant proteins, utilizing mammalian or animal cells for complex protein expression. The choice between fermentation and cell culture depends on the desired product type and the biological system best suited for efficient production.
Scale-Up and Industrial Production Considerations
Fermentation processes enable large-scale production of microorganisms or biomolecules with cost-effective substrate utilization and high product yields, making them ideal for industrial-scale manufacturing. Cell culture techniques, while offering precise control over mammalian or insect cell environments for complex protein expression, face challenges in scalability due to higher costs and stringent sterility requirements. Industrial scale-up strategies for fermentation prioritize bioreactor design and process optimization, whereas cell culture scale-up demands advanced bioreactor systems with efficient oxygen transfer and shear stress minimization to maintain cell viability and productivity.
Environmental Impact and Sustainability
Fermentation processes typically generate lower greenhouse gas emissions and consume less water compared to conventional cell culture, making them more environmentally sustainable. Cell culture often requires complex media with high energy inputs and produces significant biological waste, increasing its ecological footprint. Optimizing fermentation for bioproduction can enhance resource efficiency and reduce environmental impact in industrial biotechnology.
Future Trends in Bioprocessing Technologies
Future trends in bioprocessing technologies highlight a shift toward integrating fermentation and cell culture for enhanced bioproduction efficiency and product diversity. Advances in single-use bioreactors and continuous processing are driving scalable, cost-effective manufacturing of biologics, vaccines, and novel therapeutics. Artificial intelligence and machine learning are increasingly employed to optimize process parameters, improve yield, and ensure quality control in both fermentation and cell culture platforms.
Bioreactor design
Bioreactor design for fermentation emphasizes robust mixing and gas transfer for microbial growth, while cell culture bioreactors prioritize gentle agitation and precise environmental control to support sensitive mammalian cells.
Upstream processing
Upstream processing in fermentation utilizes microorganisms to convert substrates into desired products under controlled conditions, while cell culture involves cultivating mammalian or animal cells to produce complex biologics with precise growth requirements.
Microbial fermentation
Microbial fermentation utilizes microorganisms like bacteria and yeast to convert substrates into valuable products such as ethanol, antibiotics, and enzymes, whereas cell culture involves growing animal or plant cells under controlled conditions for biopharmaceutical and research applications.
Mammalian cell lines
Mammalian cell line fermentation optimizes protein production through controlled bioreactor environments, while traditional cell culture emphasizes cellular growth and maintenance in static or semi-static conditions.
Aerobic vs anaerobic metabolism
Fermentation primarily relies on anaerobic metabolism to produce energy without oxygen, while cell culture typically involves aerobic metabolism, using oxygen to generate energy efficiently for cell growth.
Substrate utilization
Fermentation primarily utilizes carbohydrates like glucose as substrates for microbial growth, whereas cell culture depends on complex nutrient media containing amino acids, vitamins, and glucose to support animal or plant cell proliferation.
Fed-batch cultivation
Fed-batch cultivation in fermentation optimizes microbial or cell culture growth by intermittently adding nutrients to enhance biomass and product yield without removing culture fluid.
Scale-up strategies
Scale-up strategies for fermentation emphasize optimizing bioreactor design and controlling parameters like pH, temperature, and oxygen transfer, while cell culture scale-up focuses on maintaining cell viability and function through precise control of shear stress, nutrient supply, and bioprocess monitoring systems.
Sterility assurance
Sterility assurance in fermentation relies on closed bioreactor systems with automated controls to prevent contamination, whereas cell culture requires stringent aseptic techniques and laminar flow hood environments to maintain sterile conditions.
Metabolite profiling
Fermentation enables high-throughput metabolite profiling through microbial bioprocess optimization, while cell culture provides detailed metabolomic insights in mammalian systems for therapeutic compound production.
Fermentation vs Cell culture Infographic
 njnir.com
njnir.com