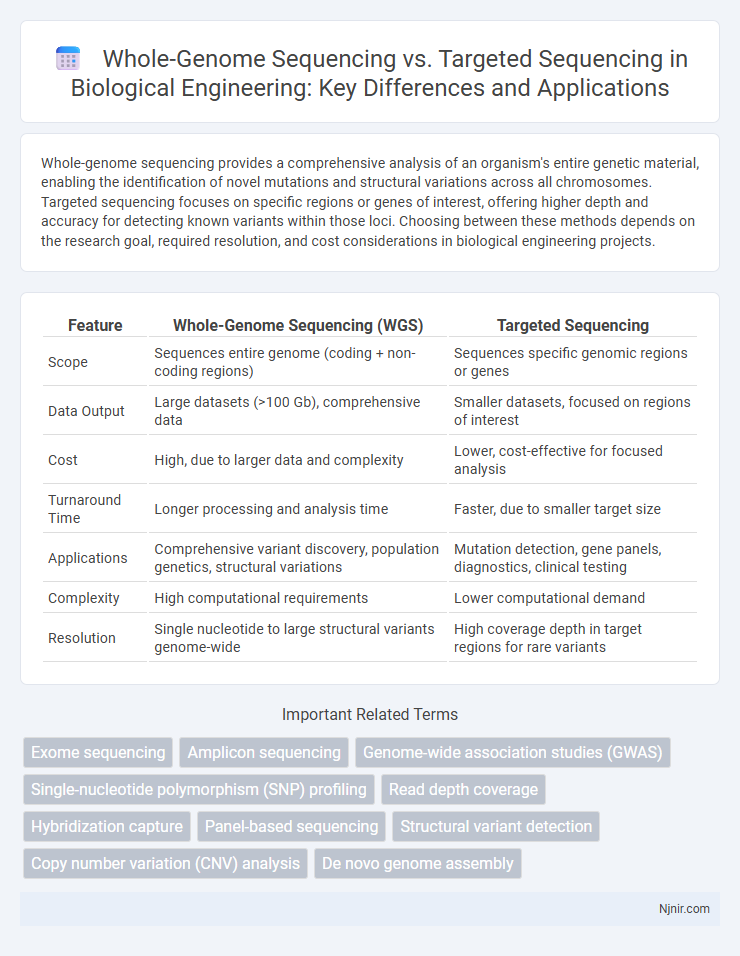
Whole-genome sequencing provides a comprehensive analysis of an organism's entire genetic material, enabling the identification of novel mutations and structural variations across all chromosomes. Targeted sequencing focuses on specific regions or genes of interest, offering higher depth and accuracy for detecting known variants within those loci. Choosing between these methods depends on the research goal, required resolution, and cost considerations in biological engineering projects.
Table of Comparison
| Feature | Whole-Genome Sequencing (WGS) | Targeted Sequencing |
|---|---|---|
| Scope | Sequences entire genome (coding + non-coding regions) | Sequences specific genomic regions or genes |
| Data Output | Large datasets (>100 Gb), comprehensive data | Smaller datasets, focused on regions of interest |
| Cost | High, due to larger data and complexity | Lower, cost-effective for focused analysis |
| Turnaround Time | Longer processing and analysis time | Faster, due to smaller target size |
| Applications | Comprehensive variant discovery, population genetics, structural variations | Mutation detection, gene panels, diagnostics, clinical testing |
| Complexity | High computational requirements | Lower computational demand |
| Resolution | Single nucleotide to large structural variants genome-wide | High coverage depth in target regions for rare variants |
Introduction to Sequencing Methods in Biological Engineering
Whole-genome sequencing (WGS) provides comprehensive analysis by decoding the entire DNA sequence of an organism, capturing all genetic variations. Targeted sequencing focuses on specific genes or regions of interest, enabling high-depth coverage and cost-effective detection of mutations within those loci. Both methods are essential in biological engineering for applications such as genetic modification, disease diagnostics, and synthetic biology, with WGS offering broad insights and targeted sequencing delivering precise, detailed information.
Principles of Whole-Genome Sequencing
Whole-genome sequencing (WGS) involves the comprehensive analysis of an organism's entire DNA sequence, capturing all genomic variations, including single nucleotide polymorphisms, insertions, deletions, and structural variants. The process relies on fragmenting the DNA into smaller pieces, sequencing these fragments using high-throughput technologies, and then assembling the sequences to reconstruct the complete genome. WGS provides an unbiased and detailed genomic landscape, enabling broad applications in disease research, evolutionary biology, and personalized medicine.
Fundamentals of Targeted Sequencing
Targeted sequencing focuses on selectively capturing and sequencing specific genomic regions of interest, enabling high-depth analysis of targeted genes or loci while reducing overall costs and data complexity compared to whole-genome sequencing (WGS). This method employs probes or primers designed to hybridize with predefined DNA segments, enhancing sensitivity for detecting rare variants, mutations, or structural changes in clinically relevant areas. Targeted sequencing provides a cost-effective, efficient approach for applications such as cancer gene panels, hereditary disease diagnosis, and pharmacogenomics by concentrating resources on critical genomic regions.
Key Differences between Whole-Genome and Targeted Sequencing
Whole-genome sequencing (WGS) analyzes the entire DNA sequence, providing comprehensive data on all genetic variations, while targeted sequencing focuses on specific genes or regions of interest, offering higher coverage and sensitivity in those areas. WGS generates extensive datasets suitable for discovering novel variants and complex structural changes, whereas targeted sequencing is cost-effective and faster, ideal for clinically relevant mutations and known hotspots. Data interpretation in WGS is more complex and resource-intensive compared to the streamlined analysis of targeted sequencing panels designed for particular genetic conditions.
Applications of Whole-Genome Sequencing in Biological Engineering
Whole-genome sequencing (WGS) enables comprehensive analysis of entire genomes, facilitating precision in genetic modification, synthetic biology, and metabolic engineering within biological engineering. It allows identification of novel genetic variants and off-target effects that targeted sequencing might miss, enhancing strain optimization and functional annotation. WGS applications include pathway engineering for biofuel production, development of genetically engineered organisms, and advancing personalized medicine through detailed genomic insights.
Targeted Sequencing: Use Cases and Benefits
Targeted sequencing focuses on analyzing specific regions of the genome, providing high coverage and accuracy for genes of interest relevant to disease diagnosis and personalized medicine. It is widely used for detecting mutations in cancer-associated genes, hereditary disorders, and pharmacogenomics applications, enabling cost-effective and faster results compared to whole-genome sequencing. By concentrating on clinically meaningful genomic areas, targeted sequencing reduces data complexity and improves interpretation efficiency in both research and clinical settings.
Data Output and Analytical Challenges
Whole-genome sequencing (WGS) generates comprehensive data covering an organism's entire genome, resulting in vast datasets often exceeding several hundred gigabases, which demand significant computational resources and storage capacity. Targeted sequencing produces smaller, more focused datasets by concentrating on specific genomic regions or gene panels, facilitating faster analysis with reduced complexity but potentially missing variants outside the targeted regions. Analytical challenges in WGS include managing the volume of raw data, ensuring accurate variant calling across repetitive sequences, and handling diverse variant types, whereas targeted sequencing challenges revolve around optimizing capture efficiency and minimizing off-target reads to maintain specificity and sensitivity.
Cost Analysis and Resource Considerations
Whole-genome sequencing typically incurs higher costs due to the extensive data generated and the need for advanced computational resources, making it less feasible for large sample sizes or budget-constrained projects. In contrast, targeted sequencing reduces expenditure by focusing on specific genomic regions, which lowers reagent use and streamlines data analysis, thus requiring fewer computational resources. Resource allocation in targeted sequencing prioritizes depth of coverage over breadth, optimizing cost-effectiveness for studies with defined genetic targets or clinical applications.
Future Trends in Genomic Sequencing Technologies
Whole-genome sequencing (WGS) offers comprehensive coverage by analyzing the entire genome, enabling broad detection of genetic variants, while targeted sequencing focuses on specific genomic regions for high-depth analysis and cost efficiency. Future trends in genomic sequencing technologies emphasize integration of long-read sequencing, enhanced accuracy with AI-driven variant interpretation, and rapid, real-time analysis to facilitate personalized medicine and large-scale population genomics. Advances in nanopore and single-molecule sequencing platforms are expected to bridge gaps between WGS and targeted approaches, optimizing both resolution and throughput for diverse clinical and research applications.
Choosing the Right Sequencing Approach for Your Research
Whole-genome sequencing (WGS) offers comprehensive analysis by capturing the entire genetic makeup, making it ideal for discovering novel variants and understanding complex traits, while targeted sequencing focuses on specific genes or regions, providing higher coverage and cost-effectiveness for studying known mutations. Selecting WGS is beneficial when broad genetic insights and detection of rare variants are crucial, whereas targeted sequencing excels in clinical diagnostics and cases with well-defined genetic targets. Consider factors such as research goals, budget constraints, required resolution, and data analysis capabilities to determine the most suitable sequencing approach.
Exome sequencing
Exome sequencing, a targeted sequencing approach covering approximately 1-2% of the genome that encodes proteins, offers a cost-effective alternative to whole-genome sequencing by focusing on clinically relevant coding regions for detecting genetic variants associated with diseases.
Amplicon sequencing
Amplicon sequencing, a targeted sequencing method, focuses on specific genomic regions to provide high-depth, cost-effective analysis compared to whole-genome sequencing, which covers the entire genome but at higher expense and lower coverage depth per region.
Genome-wide association studies (GWAS)
Whole-genome sequencing provides comprehensive variant data across the entire genome, enhancing Genome-wide association studies (GWAS) by identifying both common and rare genetic variants, whereas targeted sequencing focuses on preselected genomic regions, limiting discovery potential in GWAS analyses.
Single-nucleotide polymorphism (SNP) profiling
Whole-genome sequencing provides comprehensive SNP profiling across the entire genome, while targeted sequencing focuses on specific genomic regions, enabling higher coverage and accuracy for selected SNPs.
Read depth coverage
Whole-genome sequencing offers lower average read depth coverage across the entire genome, while targeted sequencing achieves higher read depth coverage in specific genomic regions, enhancing variant detection sensitivity.
Hybridization capture
Hybridization capture in whole-genome sequencing enables comprehensive analysis by enriching specific genomic regions, whereas targeted sequencing uses this method to selectively isolate and sequence predefined DNA segments with high accuracy.
Panel-based sequencing
Panel-based targeted sequencing offers higher coverage depth and cost-efficiency compared to whole-genome sequencing, making it ideal for detecting clinically relevant mutations in specific gene sets.
Structural variant detection
Whole-genome sequencing offers comprehensive detection of structural variants across the entire genome, whereas targeted sequencing provides higher coverage and sensitivity in specific genomic regions but may miss broader structural variations.
Copy number variation (CNV) analysis
Whole-genome sequencing provides comprehensive copy number variation (CNV) detection across the entire genome, while targeted sequencing offers higher resolution CNV analysis in specific genomic regions with greater cost-effectiveness.
De novo genome assembly
Whole-genome sequencing enables comprehensive de novo genome assembly by capturing complete genomic information, whereas targeted sequencing limits assembly to specific regions, reducing genome-wide assembly accuracy and completeness.
Whole-genome sequencing vs Targeted sequencing Infographic
 njnir.com
njnir.com