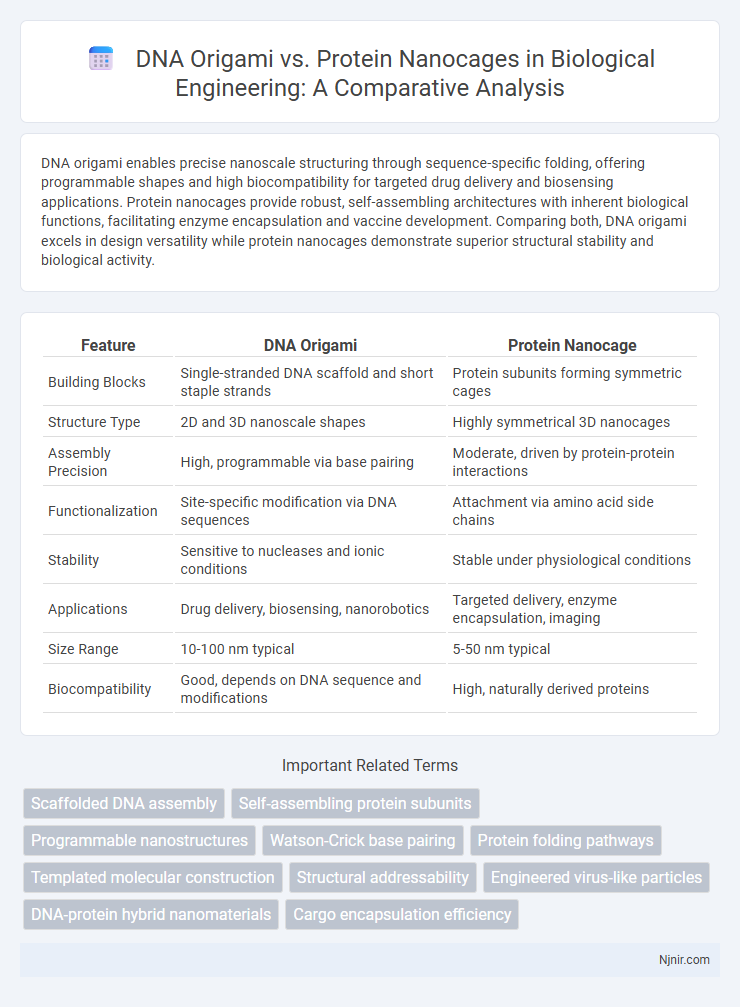
DNA origami enables precise nanoscale structuring through sequence-specific folding, offering programmable shapes and high biocompatibility for targeted drug delivery and biosensing applications. Protein nanocages provide robust, self-assembling architectures with inherent biological functions, facilitating enzyme encapsulation and vaccine development. Comparing both, DNA origami excels in design versatility while protein nanocages demonstrate superior structural stability and biological activity.
Table of Comparison
| Feature | DNA Origami | Protein Nanocage |
|---|---|---|
| Building Blocks | Single-stranded DNA scaffold and short staple strands | Protein subunits forming symmetric cages |
| Structure Type | 2D and 3D nanoscale shapes | Highly symmetrical 3D nanocages |
| Assembly Precision | High, programmable via base pairing | Moderate, driven by protein-protein interactions |
| Functionalization | Site-specific modification via DNA sequences | Attachment via amino acid side chains |
| Stability | Sensitive to nucleases and ionic conditions | Stable under physiological conditions |
| Applications | Drug delivery, biosensing, nanorobotics | Targeted delivery, enzyme encapsulation, imaging |
| Size Range | 10-100 nm typical | 5-50 nm typical |
| Biocompatibility | Good, depends on DNA sequence and modifications | High, naturally derived proteins |
Introduction to DNA Origami and Protein Nanocages
DNA origami utilizes the self-assembly properties of DNA strands to create precise nanoscale shapes and structures through complementary base pairing. Protein nanocages are naturally occurring or engineered protein assemblies that form hollow, cage-like architectures ideal for encapsulation and targeted delivery. Both DNA origami and protein nanocages offer unique advantages in nanotechnology, with DNA origami providing customizable geometric control and protein nanocages leveraging biological functionality and stability.
Molecular Design Principles
DNA origami employs the specific base-pairing rules of nucleotides to fold long single-stranded DNA into precise nanoscale shapes, leveraging Watson-Crick complementarity and programmable sequence domains for structural predictability. Protein nanocages rely on the hierarchical assembly of protein subunits through non-covalent interactions and symmetry-driven interfaces, emphasizing the folding patterns determined by amino acid sequences and protein tertiary/quaternary structures. Both molecular design principles harness self-assembly but differ fundamentally in their biomolecular building blocks and folding mechanisms, impacting stability, functionalization, and complexity of nanoscale architectures.
Structural Assembly Mechanisms
DNA origami relies on the precise folding of long single-stranded DNA scaffold strands guided by hundreds of short staple strands, enabling programmable and highly predictable two- and three-dimensional nanostructures. In contrast, protein nanocages form through the self-assembly of multiple protein subunits driven by non-covalent interactions such as hydrophobic forces, hydrogen bonding, and electrostatic interactions, often resulting in symmetrical and rigid architectures. DNA origami provides greater design flexibility and nanoscale addressability, whereas protein nanocages offer inherent biological functionality and stability in physiological conditions.
Precision and Programmability
DNA origami offers exceptional precision at the nanometer scale due to its predictable base-pairing rules, enabling the design of complex 2D and 3D shapes with high spatial resolution. Protein nanocages, while naturally assembled with inherent biological functionality, exhibit less programmability because their assembly relies on protein-protein interactions that are more challenging to engineer with atomic-level control. Advances in synthetic biology are improving the programmability of protein nanocages, but DNA origami remains superior for customizable and precise nanostructure fabrication.
Functionalization and Customization
DNA origami offers precise nanoscale functionalization through base-pair complementarity, enabling site-specific attachment of molecules with high spatial resolution. Protein nanocages provide robust structural frameworks that allow genetic or chemical modification to introduce functional groups, often enhancing stability and biological compatibility. Customization in DNA origami is highly programmable for complex shapes and dynamic behaviors, whereas protein nanocages excel in natural self-assembly and post-translational modifications for functional diversity.
Stability and Environmental Response
DNA origami structures exhibit high programmability but often face challenges in stability due to susceptibility to nucleases and environmental conditions such as pH and temperature fluctuations. Protein nanocages, composed of robust protein subunits like ferritin or virus-like particles, demonstrate enhanced stability under physiological conditions and offer responsive behavior to changes in ionic strength and redox environments. Comparative studies reveal that protein nanocages maintain structural integrity over a wider range of environmental stresses, while DNA origami requires chemical modifications or protective coatings to improve durability.
Biomedical and Therapeutic Applications
DNA origami offers precise nanoscale control for constructing complex 3D structures ideal for targeted drug delivery and biosensing in biomedical applications. Protein nanocages provide inherent biocompatibility and robust stability, making them excellent carriers for therapeutic agents and vaccines. Both platforms enhance cellular uptake and controlled release, advancing personalized medicine and immunotherapy.
Manufacturing Scalability and Cost
DNA origami offers precise nanoscale control but faces challenges in manufacturing scalability due to complex staple strand synthesis and assembly processes, leading to higher production costs. Protein nanocages benefit from biological expression systems that enable scalable and cost-effective production through fermentation and recombinant protein technology. Consequently, protein nanocages provide a more economically viable option for large-scale manufacturing compared to DNA origami structures.
Challenges and Limitations
DNA origami faces challenges including structural instability in physiological conditions and susceptibility to nucleases, limiting its in vivo applications. Protein nanocages, while offering enhanced biocompatibility and functional versatility, encounter difficulties in precise design control and potential immunogenicity. Both platforms struggle with scalability and reproducibility, constraining their practical use in nanomedicine and molecular engineering.
Future Perspectives in Biological Engineering
DNA origami offers precise nanoscale control and programmability, enabling customized molecular frameworks for targeted drug delivery and biosensing applications. Protein nanocages provide robust, biocompatible scaffolds with inherent biological functions, ideal for vaccine development and enzyme encapsulation. Future perspectives in biological engineering emphasize integrating DNA origami's design versatility with protein nanocages' functional complexity to create hybrid nanodevices for advanced therapeutics and diagnostics.
Scaffolded DNA assembly
Scaffolded DNA assembly in DNA origami enables precise nanostructure design through a long single-stranded DNA scaffold folded by short staple strands, offering higher customization and complexity compared to protein nanocages.
Self-assembling protein subunits
Self-assembling protein subunits in protein nanocages offer greater structural stability and functional versatility compared to the primarily nucleic acid-based folding mechanisms in DNA origami.
Programmable nanostructures
DNA origami offers highly programmable nanostructures with precise spatial control and versatile design capabilities, whereas protein nanocages provide robust, biologically functional architectures but with more limited programmability.
Watson-Crick base pairing
DNA origami utilizes Watson-Crick base pairing for precise nanoscale folding and assembly, enabling complex three-dimensional structures unlike protein nanocages, which rely on amino acid interactions for self-assembly.
Protein folding pathways
Protein nanocages exhibit complex protein folding pathways involving hierarchical assembly and conformational changes, whereas DNA origami relies on predictable base-pairing interactions for structural formation.
Templated molecular construction
DNA origami enables precise, programmable templated molecular construction through base-pairing specificity, whereas protein nanocages rely on protein self-assembly motifs for structurally robust but less customizable scaffolds.
Structural addressability
DNA origami offers unparalleled structural addressability through programmable base-pairing patterns, enabling precise nanoscale shape control compared to the more limited and protein-dependent architecture of protein nanocages.
Engineered virus-like particles
Engineered virus-like particles (VLPs) derived from protein nanocages offer enhanced structural stability and functional versatility compared to DNA origami, enabling precise molecular assembly for targeted drug delivery and vaccine development.
DNA-protein hybrid nanomaterials
DNA-protein hybrid nanomaterials combine the precise programmability of DNA origami with the structural stability of protein nanocages to enable advanced functionality in targeted drug delivery and nanoscale bioengineering.
Cargo encapsulation efficiency
DNA origami demonstrates higher cargo encapsulation efficiency compared to protein nanocages due to its programmable structural precision and customizable binding sites.
DNA origami vs Protein nanocage Infographic
 njnir.com
njnir.com