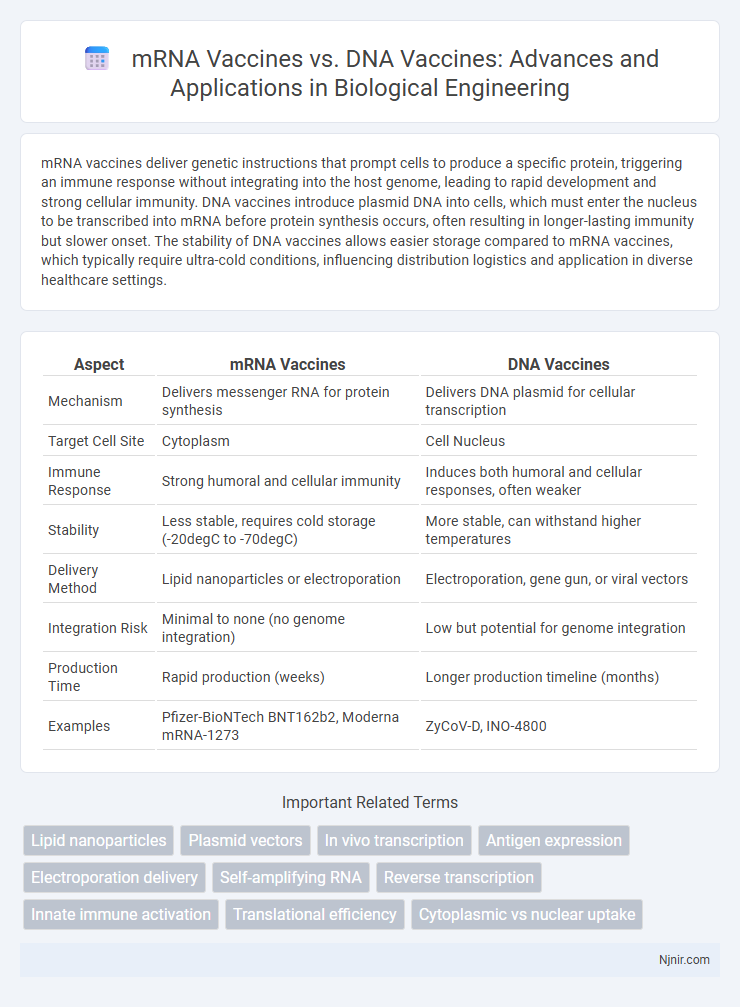
mRNA vaccines deliver genetic instructions that prompt cells to produce a specific protein, triggering an immune response without integrating into the host genome, leading to rapid development and strong cellular immunity. DNA vaccines introduce plasmid DNA into cells, which must enter the nucleus to be transcribed into mRNA before protein synthesis occurs, often resulting in longer-lasting immunity but slower onset. The stability of DNA vaccines allows easier storage compared to mRNA vaccines, which typically require ultra-cold conditions, influencing distribution logistics and application in diverse healthcare settings.
Table of Comparison
| Aspect | mRNA Vaccines | DNA Vaccines |
|---|---|---|
| Mechanism | Delivers messenger RNA for protein synthesis | Delivers DNA plasmid for cellular transcription |
| Target Cell Site | Cytoplasm | Cell Nucleus |
| Immune Response | Strong humoral and cellular immunity | Induces both humoral and cellular responses, often weaker |
| Stability | Less stable, requires cold storage (-20degC to -70degC) | More stable, can withstand higher temperatures |
| Delivery Method | Lipid nanoparticles or electroporation | Electroporation, gene gun, or viral vectors |
| Integration Risk | Minimal to none (no genome integration) | Low but potential for genome integration |
| Production Time | Rapid production (weeks) | Longer production timeline (months) |
| Examples | Pfizer-BioNTech BNT162b2, Moderna mRNA-1273 | ZyCoV-D, INO-4800 |
Overview of mRNA and DNA Vaccines
mRNA vaccines use messenger RNA to instruct cells to produce antigens, triggering an immune response without using live virus, while DNA vaccines deliver plasmid DNA encoding antigens directly into the nucleus for protein synthesis. mRNA vaccines, such as Pfizer-BioNTech and Moderna, benefit from rapid development and strong cellular immunity, whereas DNA vaccines, like ZyCoV-D, offer stability and ease of storage but require delivery methods like electroporation. Both platforms represent innovative approaches to immunization with distinct mechanisms influencing efficacy, production, and distribution.
Mechanisms of Action: mRNA vs DNA Vaccines
mRNA vaccines deliver synthetic messenger RNA into host cells, instructing ribosomes to produce specific viral proteins that trigger an immune response without entering the cell nucleus. DNA vaccines introduce plasmid DNA into the host cell nucleus, where it is transcribed into mRNA before protein translation occurs in the cytoplasm, eliciting immunity. Both approaches activate adaptive immune responses by presenting antigens, but mRNA vaccines bypass nuclear entry, potentially offering faster and more transient protein expression.
Delivery Systems and Formulation Technologies
mRNA vaccines utilize lipid nanoparticle delivery systems to protect the fragile mRNA molecules and facilitate cellular uptake, ensuring efficient protein translation within the cytoplasm. DNA vaccines primarily rely on electroporation or viral vectors to deliver plasmid DNA into the cell nucleus, where transcription occurs before protein synthesis. Advanced formulation technologies for mRNA include ionizable lipids and polyethylene glycol (PEG)-lipids, enhancing stability and reducing immunogenicity, whereas DNA vaccine formulations focus on plasmid optimization and adjuvants to boost immune response and expression levels.
Immune Response: Efficacy and Duration
mRNA vaccines induce a strong cellular and humoral immune response by delivering synthetic mRNA encoding the antigen directly into the cytoplasm, resulting in rapid protein production and robust activation of T cells and B cells. DNA vaccines introduce plasmid DNA into the cell nucleus, requiring transcription before antigen expression, which may lead to a comparatively slower and sometimes less potent immune response. Generally, mRNA vaccines demonstrate higher efficacy and longer-lasting immunity due to efficient antigen presentation and strong immunogenicity, making them highly effective against viral infections like SARS-CoV-2.
Safety Profiles and Side Effects
mRNA vaccines, such as Pfizer-BioNTech and Moderna, have demonstrated a strong safety profile with primarily mild to moderate side effects like injection site pain, fatigue, and headache. DNA vaccines, although less widely administered, show similar tolerability but may involve different delivery methods such as electroporation, which can influence side effect frequency and severity. Both vaccine types generally avoid serious adverse events, with ongoing surveillance confirming their safety in large populations.
Manufacturing and Scalability
mRNA vaccines benefit from rapid and flexible manufacturing processes, allowing for swift adaptation to emerging variants with standardized cell-free synthesis techniques. DNA vaccines typically require more complex production involving bacterial fermentation and plasmid purification, which can extend manufacturing timelines and scale-up challenges. The scalability of mRNA vaccines is enhanced by modular production platforms that support high-throughput output, whereas DNA vaccine production demands stringent quality control steps to ensure plasmid stability and purity.
Storage and Distribution Requirements
mRNA vaccines require ultra-cold storage conditions, typically between -80degC to -20degC, necessitating specialized freezers and cold chain logistics for effective distribution. DNA vaccines are generally more stable at higher temperatures, often stored at standard refrigeration temperatures of 2degC to 8degC, simplifying storage and transportation requirements. These differences significantly impact the scalability and accessibility of vaccine deployment, especially in low-resource settings.
Clinical Trials and Approved Applications
mRNA vaccines have shown rapid development and high efficacy in clinical trials, most notably with the Pfizer-BioNTech and Moderna COVID-19 vaccines receiving emergency use authorization and full approval globally. DNA vaccines, while still under investigation in clinical trials for diseases such as Zika and HPV, have fewer approved applications, with notable approvals in veterinary medicine rather than widespread human use. Comparative data emphasize mRNA vaccine platforms' accelerated immune response and scalability, whereas DNA vaccines require optimization for delivery methods and sustained expression in target cells.
Challenges and Limitations
mRNA vaccines face challenges such as instability requiring ultra-cold storage and limited long-term safety data due to their novel technology. DNA vaccines encounter limitations including lower immunogenicity in humans and reliance on efficient delivery methods like electroporation for cellular uptake. Both types must address potential regulatory hurdles and scalability issues for widespread implementation.
Future Perspectives in Vaccine Development
mRNA vaccines exhibit rapid development cycles and high adaptability to emerging variants, positioning them as a cornerstone in future vaccine innovation. DNA vaccines offer advantages in stability and ease of manufacturing, making them promising candidates for large-scale immunization programs. Advances in delivery technologies and adjuvants are expected to enhance the efficacy and safety profiles of both vaccine platforms, accelerating their integration into next-generation immunization strategies.
Lipid nanoparticles
Lipid nanoparticles enhance the delivery efficiency and stability of mRNA vaccines by facilitating cellular uptake and protecting the nucleic acid, whereas DNA vaccines often require alternative delivery methods like electroporation due to their larger plasmid size and different cellular entry mechanisms.
Plasmid vectors
mRNA vaccines utilize lipid nanoparticle delivery without plasmid vectors, whereas DNA vaccines rely on plasmid vectors to deliver genetic material encoding antigens into host cells for immune response activation.
In vivo transcription
mRNA vaccines enable direct in vivo protein synthesis by delivering messenger RNA to the cytoplasm, whereas DNA vaccines require nuclear entry for transcription into mRNA before protein translation occurs.
Antigen expression
mRNA vaccines enable rapid, high-level antigen expression directly in the cytoplasm without nuclear entry, while DNA vaccines require nuclear localization for transcription before antigen production, often resulting in slower and sometimes lower antigen expression.
Electroporation delivery
Electroporation delivery enhances DNA vaccine uptake by creating temporary cell membrane pores, significantly improving immune response efficiency compared to mRNA vaccines, which typically utilize lipid nanoparticles for delivery.
Self-amplifying RNA
Self-amplifying RNA vaccines enhance mRNA vaccine efficacy by enabling intracellular RNA replication, resulting in higher antigen expression compared to conventional DNA vaccines that require nuclear entry for transcription.
Reverse transcription
mRNA vaccines do not undergo reverse transcription into DNA, unlike some DNA vaccines that carry a theoretical risk of integration into the host genome.
Innate immune activation
mRNA vaccines rapidly activate innate immune responses through pattern recognition receptors like Toll-like receptors, enhancing antigen presentation, while DNA vaccines elicit a comparatively moderate innate response primarily via cytosolic DNA sensors such as cGAS-STING pathways.
Translational efficiency
mRNA vaccines exhibit higher translational efficiency than DNA vaccines due to their direct cytoplasmic translation without requiring nuclear entry, leading to faster and more robust protein expression.
Cytoplasmic vs nuclear uptake
mRNA vaccines utilize cytoplasmic uptake for direct protein translation, whereas DNA vaccines require nuclear uptake to enable transcription before antigen expression.
mRNA vaccines vs DNA vaccines Infographic
 njnir.com
njnir.com