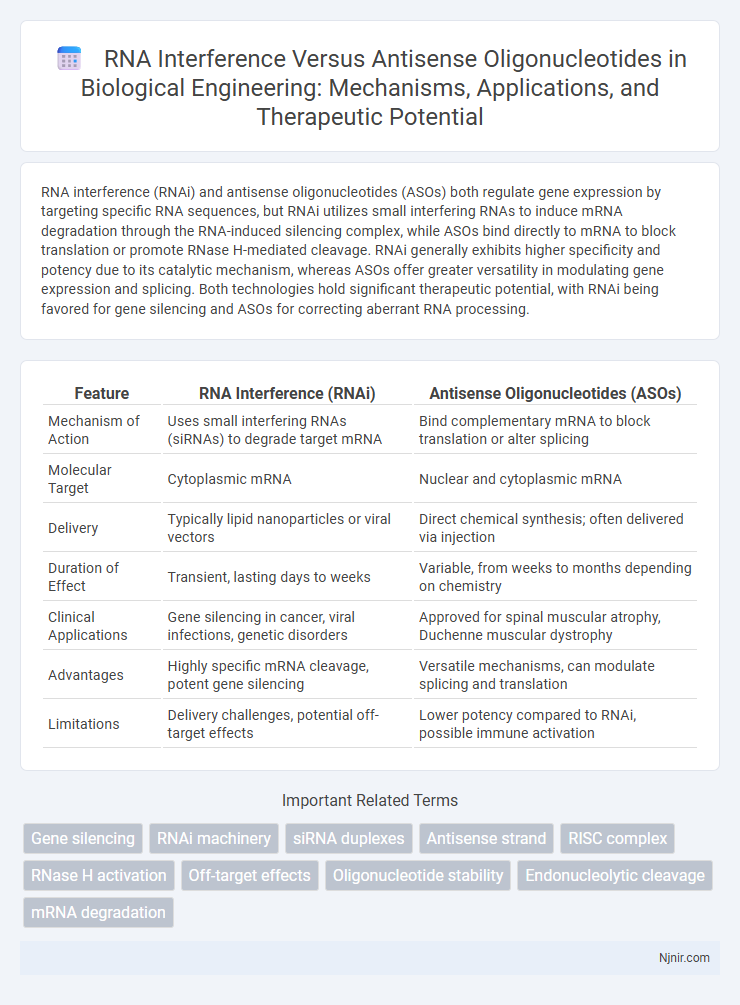
RNA interference (RNAi) and antisense oligonucleotides (ASOs) both regulate gene expression by targeting specific RNA sequences, but RNAi utilizes small interfering RNAs to induce mRNA degradation through the RNA-induced silencing complex, while ASOs bind directly to mRNA to block translation or promote RNase H-mediated cleavage. RNAi generally exhibits higher specificity and potency due to its catalytic mechanism, whereas ASOs offer greater versatility in modulating gene expression and splicing. Both technologies hold significant therapeutic potential, with RNAi being favored for gene silencing and ASOs for correcting aberrant RNA processing.
Table of Comparison
| Feature | RNA Interference (RNAi) | Antisense Oligonucleotides (ASOs) |
|---|---|---|
| Mechanism of Action | Uses small interfering RNAs (siRNAs) to degrade target mRNA | Bind complementary mRNA to block translation or alter splicing |
| Molecular Target | Cytoplasmic mRNA | Nuclear and cytoplasmic mRNA |
| Delivery | Typically lipid nanoparticles or viral vectors | Direct chemical synthesis; often delivered via injection |
| Duration of Effect | Transient, lasting days to weeks | Variable, from weeks to months depending on chemistry |
| Clinical Applications | Gene silencing in cancer, viral infections, genetic disorders | Approved for spinal muscular atrophy, Duchenne muscular dystrophy |
| Advantages | Highly specific mRNA cleavage, potent gene silencing | Versatile mechanisms, can modulate splicing and translation |
| Limitations | Delivery challenges, potential off-target effects | Lower potency compared to RNAi, possible immune activation |
Overview of Gene Silencing Technologies
RNA interference (RNAi) is a biological process where small interfering RNA (siRNA) molecules guide the RNA-induced silencing complex (RISC) to degrade complementary mRNA, effectively silencing target gene expression. Antisense oligonucleotides (ASOs) are short, synthetic strands of nucleotides designed to bind specifically to target mRNA sequences, preventing translation or altering splicing patterns. Both technologies enable post-transcriptional gene silencing but differ in their mechanisms, with RNAi relying on RISC-mediated cleavage and ASOs functioning through steric hindrance or RNase H recruitment.
Mechanisms of RNA Interference (RNAi)
RNA interference (RNAi) operates through a highly specific mechanism where small interfering RNAs (siRNAs) guide the RNA-induced silencing complex (RISC) to complementary mRNA, leading to its degradation and subsequent gene silencing. In contrast, antisense oligonucleotides (ASOs) primarily bind to target mRNA to alter splicing or block translation without necessarily inducing mRNA cleavage. The RNAi pathway involves Dicer-mediated processing of double-stranded RNA into siRNAs, which is a critical step distinguishing RNAi from the direct hybridization approach of ASOs.
Mechanisms of Antisense Oligonucleotides (ASOs)
Antisense oligonucleotides (ASOs) function by binding specifically to target RNA sequences through complementary base pairing, leading to the modulation of gene expression. They exert their effects by promoting RNase H-mediated cleavage of the RNA strand, blocking ribosomal translation, or altering splicing patterns to restore or disrupt protein production. This precise mechanism enables ASOs to target disease-causing mRNAs, offering therapeutic potential distinct from RNA interference pathways that primarily rely on the RNA-induced silencing complex (RISC) for mRNA degradation.
Molecular Design and Structure Comparison
RNA interference (RNAi) utilizes short double-stranded RNA molecules, such as siRNAs, that guide the RNA-induced silencing complex (RISC) to degrade complementary mRNA, featuring a duplex structure with 19-21 base pairs and characteristic 2-nucleotide 3' overhangs. Antisense oligonucleotides (ASOs) are single-stranded DNA or RNA analogs engineered to bind target mRNA via Watson-Crick base pairing, typically 15-25 nucleotides long, often chemically modified with phosphorothioate backbones and 2'-O-methyl or locked nucleic acid (LNA) modifications to enhance stability and affinity. The molecular design of RNAi emphasizes double-stranded integrity and precise siRNA sequence to ensure efficient RISC loading, whereas ASO design focuses on single-strand conformation and chemical modifications to improve nuclease resistance and target specificity.
Cellular Uptake and Delivery Systems
RNA interference (RNAi) utilizes small interfering RNAs (siRNAs) that require efficient cellular uptake mediated by lipid nanoparticles or viral vectors to achieve gene silencing. Antisense oligonucleotides (ASOs) often employ modified chemical backbones to enhance stability and are delivered through conjugation with cell-penetrating peptides or lipid-based carriers for optimal intracellular access. Both RNAi and ASO delivery systems face challenges in endosomal escape and targeted tissue distribution, necessitating advanced nanoparticle engineering for improved therapeutic outcomes.
Specificity and Off-Target Effects
RNA interference (RNAi) provides high specificity by utilizing small interfering RNAs (siRNAs) or microRNAs (miRNAs) that guide the RNA-induced silencing complex (RISC) to degrade target mRNA sequences, yet it can cause off-target effects due to partial complementarity with non-target transcripts. Antisense oligonucleotides (ASOs) exhibit specificity through Watson-Crick base pairing to target mRNA, often leading to RNase H-mediated degradation or steric blockade, but off-target hybridization and unintended gene modulation remain challenges. Advances in chemical modifications and design algorithms improve the target affinity and reduce off-target interactions for both RNAi and ASOs, enhancing therapeutic precision in gene silencing applications.
Therapeutic Applications and FDA Approvals
RNA interference (RNAi) and antisense oligonucleotides (ASOs) offer promising therapeutic approaches by selectively silencing disease-causing genes at the mRNA level. RNAi-based therapies, such as patisiran (Onpattro), have gained FDA approval for treating hereditary transthyretin-mediated amyloidosis, while ASO drugs like nusinersen (Spinraza) are FDA-approved for spinal muscular atrophy, highlighting their clinical potential in genetic and neuromuscular disorders. Both modalities provide targeted gene modulation with ongoing advancements aiming to expand applications in oncology, viral infections, and rare genetic diseases.
Challenges and Limitations of RNAi
RNA interference (RNAi) faces significant challenges including off-target effects, immune stimulation, and difficulties in efficient delivery to target cells. RNAi mechanisms often struggle with instability in biological fluids and unintended gene silencing, limiting therapeutic applications compared to Antisense oligonucleotides (ASOs), which offer more precise gene targeting and better pharmacokinetic profiles. Furthermore, RNAi's reliance on the RNA-induced silencing complex (RISC) can introduce variability in silencing efficiency across different tissues.
Challenges and Limitations of ASOs
Antisense oligonucleotides (ASOs) face challenges including off-target effects, limited cellular uptake, and potential immunogenicity, which can reduce therapeutic efficacy and safety. Unlike RNA interference (RNAi), which utilizes the RNA-induced silencing complex (RISC) for targeted mRNA degradation, ASOs rely on RNase H-mediated cleavage or steric hindrance, limiting their mechanism versatility. The stability and delivery of ASOs to specific tissues remain critical hurdles, often requiring chemical modifications and advanced delivery systems to enhance bioavailability and reduce rapid degradation.
Future Perspectives in Gene Silencing Technologies
Future perspectives in gene silencing technologies highlight RNA interference (RNAi) and antisense oligonucleotides (ASOs) as promising tools for precise gene modulation. Advances in delivery systems, such as lipid nanoparticles and conjugated ligands, aim to improve tissue specificity and reduce off-target effects. Emerging research focuses on enhancing the stability and potency of these molecules to expand therapeutic applications across genetic disorders, cancer, and viral infections.
Gene silencing
RNA interference achieves gene silencing by degrading target mRNA through the RNA-induced silencing complex, while antisense oligonucleotides block gene expression by binding complementary mRNA sequences to inhibit translation or alter splicing.
RNAi machinery
RNA interference utilizes the RNA-induced silencing complex (RISC) machinery to degrade target mRNA, whereas antisense oligonucleotides modulate gene expression by sterically blocking or inducing RNase H-mediated cleavage without engaging RISC.
siRNA duplexes
siRNA duplexes in RNA interference offer precise gene silencing by promoting mRNA degradation, whereas antisense oligonucleotides primarily inhibit gene expression through steric blocking or RNase H-mediated cleavage.
Antisense strand
Antisense oligonucleotides specifically bind to the antisense strand of RNA to modulate gene expression by preventing translation or altering splicing, distinguishing them from RNA interference mechanisms that primarily target the sense strand through siRNA incorporation.
RISC complex
RNA interference utilizes the RISC complex to guide sequence-specific cleavage of target mRNAs, whereas antisense oligonucleotides generally function through RNase H-mediated degradation without involving RISC.
RNase H activation
RNA interference utilizes the RNA-induced silencing complex (RISC) to degrade target mRNA without RNase H activation, whereas antisense oligonucleotides commonly trigger RNase H-mediated cleavage to suppress gene expression.
Off-target effects
RNA interference exhibits higher off-target effects due to microRNA-like seed region interactions, whereas antisense oligonucleotides demonstrate reduced off-target binding by targeting specific RNA sequences with higher specificity.
Oligonucleotide stability
RNA interference (RNAi) techniques typically exhibit enhanced oligonucleotide stability through the use of chemically modified siRNAs, whereas antisense oligonucleotides (ASOs) require extensive backbone and sugar modifications to achieve comparable resistance to nuclease degradation.
Endonucleolytic cleavage
RNA interference mediates precise endonucleolytic cleavage of target mRNA through the RISC complex, whereas antisense oligonucleotides primarily induce target RNA degradation via RNase H-mediated cleavage without RISC involvement.
mRNA degradation
RNA interference utilizes siRNA or miRNA to induce mRNA degradation via the RISC complex, whereas antisense oligonucleotides bind complementary mRNA sequences to trigger RNase H-mediated degradation or sterically block translation.
RNA interference vs Antisense oligonucleotides Infographic
 njnir.com
njnir.com