Microfluidics enables precise control and manipulation of fluids at the microscale, facilitating cellular analysis and high-throughput screening in biological engineering. Organ-on-chip technology integrates microfluidic systems with living cells to replicate organ-level functions, offering advanced platforms for disease modeling and drug testing. The combination of microfluidics and organ-on-chip devices drives innovation by enhancing physiological relevance and improving experimental accuracy.
Table of Comparison
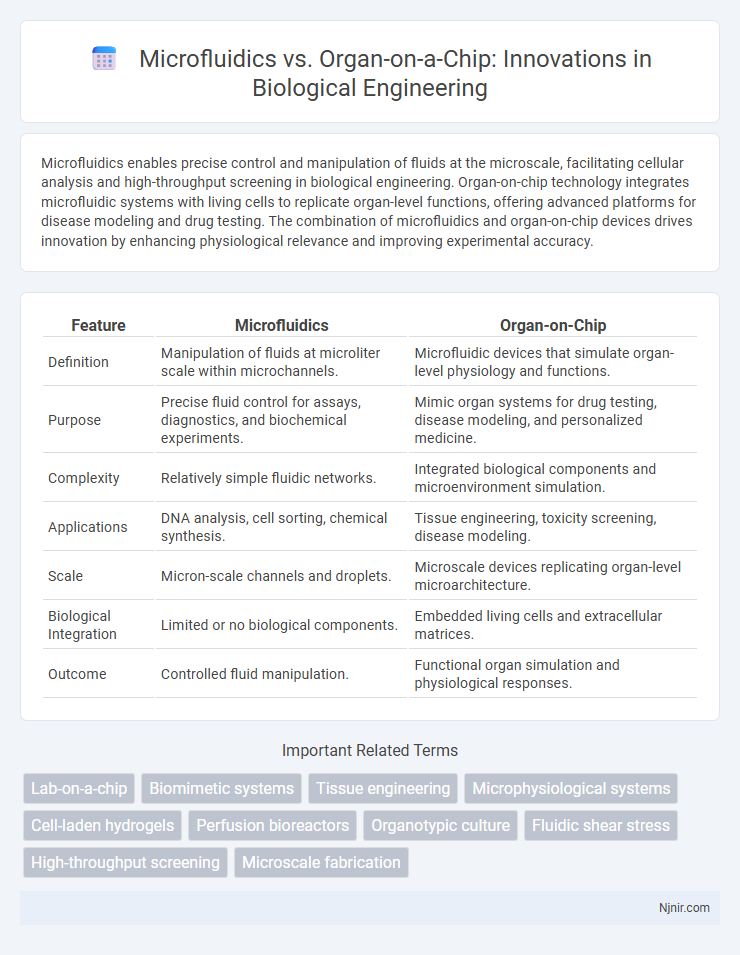
| Feature | Microfluidics | Organ-on-Chip |
|---|---|---|
| Definition | Manipulation of fluids at microliter scale within microchannels. | Microfluidic devices that simulate organ-level physiology and functions. |
| Purpose | Precise fluid control for assays, diagnostics, and biochemical experiments. | Mimic organ systems for drug testing, disease modeling, and personalized medicine. |
| Complexity | Relatively simple fluidic networks. | Integrated biological components and microenvironment simulation. |
| Applications | DNA analysis, cell sorting, chemical synthesis. | Tissue engineering, toxicity screening, disease modeling. |
| Scale | Micron-scale channels and droplets. | Microscale devices replicating organ-level microarchitecture. |
| Biological Integration | Limited or no biological components. | Embedded living cells and extracellular matrices. |
| Outcome | Controlled fluid manipulation. | Functional organ simulation and physiological responses. |
Introduction to Microfluidics and Organ-on-Chip Technologies
Microfluidics involves manipulating fluids at the microscale to enable precise control over chemical and biological processes, essential for diagnostics and drug development. Organ-on-chip technology integrates microfluidic channels with living cells to recreate the physiological functions of human organs, providing advanced models for biomedical research. These platforms enhance experimental accuracy and reduce reliance on animal testing by simulating dynamic organ environments in vitro.
Core Principles and Mechanisms
Microfluidics involves manipulating fluids at the microscale to precisely control chemical and biological environments through channels typically sized between 10 and 100 micrometers, enabling high-throughput analysis and reduced reagent consumption. Organ-on-chip technology builds upon microfluidics by integrating living cells within microengineered environments that mimic the physiological functions of human tissues or organs, utilizing mechanical forces and fluid flow to replicate in vivo conditions. Core mechanisms in microfluidics rely on laminar flow and diffusion-dominated transport, whereas organ-on-chip devices incorporate dynamic stimuli such as shear stress, cyclic stretching, and biochemical gradients to simulate organ-level responses.
Material Selection and Device Fabrication
Microfluidics primarily utilizes polymers such as polydimethylsiloxane (PDMS) for rapid prototyping and cost-effective device fabrication, while organ-on-chip technologies often require biocompatible materials like cyclic olefin copolymer (COC) or glass to support cell viability and mimic physiological environments. Fabrication of microfluidic devices frequently involves soft lithography and injection molding, whereas organ-on-chip systems integrate microfabrication techniques with microenvironmental control elements like microvalves and sensors. Material selection in organ-on-chip platforms emphasizes mechanical properties, gas permeability, and optical transparency to ensure accurate biological modeling and real-time observation.
Advantages and Limitations of Microfluidics
Microfluidics offers precise control of fluids at the microscale, enabling high-throughput screening and reduced reagent consumption, which significantly decreases experimental costs. Its limitations include challenges in fabricating complex 3D tissue models and replicating the dynamic environment of living organs, aspects where organ-on-chip technology excels by mimicking physiological functions more accurately. Despite this, microfluidics remains essential for fundamental studies and integration in organ-on-chip platforms due to its scalability and versatility.
Organ-on-Chip: Mimicking Human Physiology
Organ-on-chip technology advances microfluidics by integrating living human cells within microengineered environments, replicating the mechanical and biochemical functions of specific organs. These microfluidic devices simulate complex tissue interfaces and physiological responses, enabling precise modeling of human pathophysiology and drug interactions. Organ-on-chip platforms significantly enhance predictive accuracy for disease modeling and personalized medicine compared to traditional microfluidic systems.
Applications in Drug Discovery and Testing
Microfluidics enables precise manipulation of fluids at the microscale, facilitating high-throughput screening and drug delivery studies essential for early-stage drug discovery. Organ-on-chip technology integrates microfluidic channels with living cells to mimic organ-level functions and physiological responses, providing more accurate models for drug toxicity and efficacy testing. These technologies combined significantly enhance predictive accuracy in preclinical drug development, reducing reliance on animal models and accelerating the transition to clinical trials.
Integration with Biosensors and Data Analysis
Microfluidics technology enables precise manipulation of fluids at the microscale, facilitating real-time integration with biosensors for continuous monitoring of biological parameters. Organ-on-chip systems leverage microfluidic platforms to mimic physiological environments while incorporating embedded biosensors that generate high-resolution data on cellular responses. Advanced data analysis algorithms process this sensor data to provide insights into dynamic biological processes, enhancing drug testing and disease modeling accuracy.
Scalability and Commercialization Challenges
Microfluidics technology enables precise manipulation of fluids at the microscale, driving advancements in organ-on-chip platforms that simulate human physiology for drug testing. Scalability issues arise due to complex fabrication processes and integration of multiple cell types, limiting mass production and consistent quality in organ-on-chip devices. Commercialization challenges include high manufacturing costs, regulatory hurdles, and the need for standardized protocols to ensure reproducibility and broader adoption in pharmaceutical and biomedical industries.
Future Trends in Biological Engineering
Microfluidics technology is advancing rapidly, enabling precise manipulation of fluids at the microscale for biological applications. Organ-on-chip platforms integrate microfluidic systems with living cells to simulate complex organ functions, drastically enhancing disease modeling and drug testing. Future trends emphasize enhanced biomimicry, real-time monitoring, and integration with artificial intelligence to revolutionize personalized medicine and regenerative therapies.
Conclusion: Comparative Analysis and Outlook
Microfluidics offers precision control of fluid dynamics at microscale, enabling high-throughput screening and detailed cellular studies, while organ-on-chip systems provide physiologically relevant models that mimic complex tissue interfaces and organ functions. The convergence of these technologies drives advancements in personalized medicine and drug development by combining microfluidic engineering with biomimetic tissue models. Future research prioritizes enhancing integration, scalability, and standardization to bridge the gap between in vitro experimentation and clinical applications effectively.
Lab-on-a-chip
Lab-on-a-chip technology integrates microfluidics to miniaturize and automate biological assays, while organ-on-chip platforms utilize microfluidic systems to replicate organ-level functions for advanced disease modeling and drug testing.
Biomimetic systems
Microfluidics enables precise manipulation of fluids at the microscale, forming the foundational technology for organ-on-chip devices that replicate biomimetic systems by mimicking physiological microenvironments and tissue interfaces for advanced biomedical research.
Tissue engineering
Organ-on-chip technology leverages microfluidics to create dynamic, biomimetic environments that enhance tissue engineering by precisely controlling cellular microenvironments and fluid flow at microscale levels.
Microphysiological systems
Microphysiological systems integrate microfluidics and organ-on-chip technologies to create complex, biomimetic models of human tissue functions for advanced drug testing and disease research.
Cell-laden hydrogels
Cell-laden hydrogels in microfluidics enable precise control of cellular microenvironments, while organ-on-chip platforms integrate these hydrogels to replicate tissue-specific physiological functions for advanced biomedical applications.
Perfusion bioreactors
Perfusion bioreactors enhance microfluidics and organ-on-chip systems by providing continuous nutrient flow and waste removal, improving cell viability and mimicking physiological shear stress for more accurate tissue modeling.
Organotypic culture
Organ-on-chip technology enhances organotypic culture by integrating microfluidic systems to replicate physiological microenvironments and improve tissue-level functionality.
Fluidic shear stress
Microfluidics precisely controls fluidic shear stress to replicate physiological conditions, while organ-on-chip devices integrate microfluidic systems to simulate tissue-specific shear stress for enhanced in vitro modeling of cellular responses.
High-throughput screening
Microfluidics enables high-throughput screening by precisely manipulating small fluid volumes, while organ-on-chip technology integrates microfluidic systems with living cell cultures to mimic organ-level functions for more physiologically relevant drug testing.
Microscale fabrication
Microscale fabrication techniques in microfluidics enable precise manipulation of fluids at the micron scale, while organ-on-chip technology leverages these methods to replicate complex tissue architectures and dynamic physiological environments for advanced biomedical applications.
Microfluidics vs Organ-on-chip Infographic
 njnir.com
njnir.com