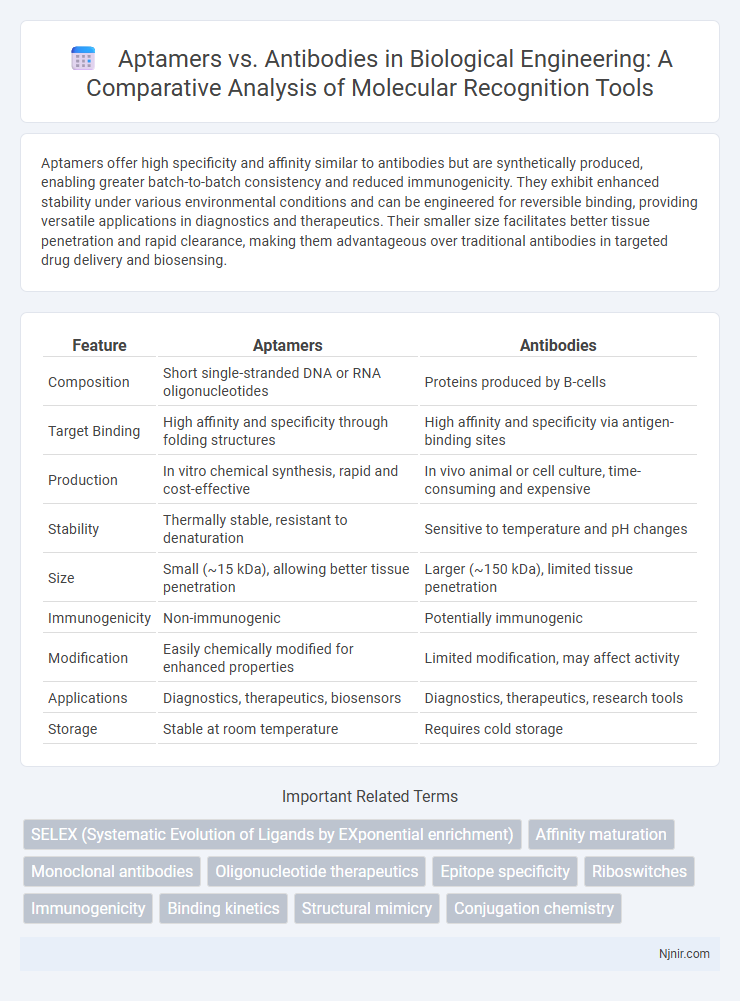
Aptamers offer high specificity and affinity similar to antibodies but are synthetically produced, enabling greater batch-to-batch consistency and reduced immunogenicity. They exhibit enhanced stability under various environmental conditions and can be engineered for reversible binding, providing versatile applications in diagnostics and therapeutics. Their smaller size facilitates better tissue penetration and rapid clearance, making them advantageous over traditional antibodies in targeted drug delivery and biosensing.
Table of Comparison
| Feature | Aptamers | Antibodies |
|---|---|---|
| Composition | Short single-stranded DNA or RNA oligonucleotides | Proteins produced by B-cells |
| Target Binding | High affinity and specificity through folding structures | High affinity and specificity via antigen-binding sites |
| Production | In vitro chemical synthesis, rapid and cost-effective | In vivo animal or cell culture, time-consuming and expensive |
| Stability | Thermally stable, resistant to denaturation | Sensitive to temperature and pH changes |
| Size | Small (~15 kDa), allowing better tissue penetration | Larger (~150 kDa), limited tissue penetration |
| Immunogenicity | Non-immunogenic | Potentially immunogenic |
| Modification | Easily chemically modified for enhanced properties | Limited modification, may affect activity |
| Applications | Diagnostics, therapeutics, biosensors | Diagnostics, therapeutics, research tools |
| Storage | Stable at room temperature | Requires cold storage |
Introduction to Aptamers and Antibodies
Aptamers are short, single-stranded nucleic acids that fold into unique three-dimensional structures, enabling them to bind targets with high specificity and affinity, similar to antibodies. Antibodies are Y-shaped proteins produced by the immune system, widely used in diagnostics and therapeutics due to their ability to recognize and bind antigens precisely. Both aptamers and antibodies serve as molecular recognition elements, but aptamers offer advantages such as chemical synthesis, thermal stability, and low immunogenicity.
Structural Features of Aptamers and Antibodies
Aptamers are short, single-stranded nucleic acids that fold into unique three-dimensional shapes, enabling high specificity and affinity for their targets through structural motifs such as loops, stems, and bulges. Antibodies possess a Y-shaped protein structure composed of two heavy and two light chains forming variable and constant regions, with the variable regions containing complementarity-determining regions (CDRs) responsible for antigen binding. While aptamers exhibit flexible conformations and smaller size allowing better tissue penetration, antibodies have larger, more complex structures providing robust stability and diverse effector functions.
Mechanisms of Target Recognition
Aptamers recognize targets through specific three-dimensional folding that forms unique binding pockets for high-affinity interaction with molecules such as proteins, peptides, or small compounds. Antibodies bind antigens via variable regions in their Fab fragments, with complementarity-determining regions (CDRs) providing precise molecular recognition driven by non-covalent interactions like hydrogen bonds and van der Waals forces. The versatility of aptamers allows for conformational adaptability and reversible target binding, whereas antibodies rely on stable protein-protein interactions shaped by somatic hypermutation and affinity maturation.
Synthesis and Production Methods
Aptamers are synthesized using automated chemical processes such as SELEX (Systematic Evolution of Ligands by EXponential enrichment), allowing precise control and rapid production with high reproducibility. Antibodies are produced biologically through immunization of animals or hybridoma technology, which involves complex cell culture systems and longer development times. The synthetic nature of aptamers enables scalable manufacturing and modifications, whereas antibody production depends on living systems, making it more variable and cost-intensive.
Binding Affinity and Specificity
Aptamers exhibit high binding affinity with dissociation constants (Kd) typically in the low nanomolar to picomolar range, comparable to or exceeding that of antibodies. Their specificity is driven by unique three-dimensional folding, enabling selective binding to small molecules, proteins, and cells with minimal cross-reactivity. Unlike antibodies, aptamers offer advantages of in vitro selection, chemical synthesis, and reduced immunogenicity, enhancing their potential in diagnostic and therapeutic applications.
Stability and Storage Considerations
Aptamers exhibit superior stability compared to antibodies, maintaining functional integrity under a wider range of temperatures and pH levels, which simplifies storage and transport conditions. Unlike antibodies that require cold chain storage typically between 2-8degC to preserve their activity, aptamers can often be stored at room temperature or in lyophilized form without significant loss of binding affinity. This robustness in stability reduces logistical challenges and costs associated with long-term storage and handling in diagnostic and therapeutic applications.
Applications in Diagnostics
Aptamers offer high specificity and stability in diagnostic applications, enabling sensitive detection of biomarkers in complex biological samples with minimal batch-to-batch variability. Unlike antibodies, aptamers can be synthesized chemically, ensuring consistent quality and facilitating rapid modifications for enhanced target binding and multiplexing capabilities in biosensors and point-of-care devices. Their ability to bind a wide range of targets, including proteins, small molecules, and cells, makes aptamers versatile tools for early disease detection and real-time monitoring in clinical diagnostics.
Therapeutic Potential and Use Cases
Aptamers offer significant therapeutic potential due to their high specificity, low immunogenicity, and ease of synthesis, making them ideal for targeted drug delivery and diagnostic applications. Unlike antibodies, aptamers demonstrate rapid tissue penetration and reversible binding, which enhances their use in cancer therapy, antiviral treatments, and biosensors. Current clinical trials highlight aptamers' versatility in treating conditions such as macular degeneration, thrombosis, and inflammatory diseases, positioning them as promising alternatives to traditional antibody-based therapies.
Cost-Effectiveness and Scalability
Aptamers offer superior cost-effectiveness compared to antibodies due to their synthetic production process, which eliminates the need for animals and reduces batch-to-batch variability. Their scalable chemical synthesis allows for rapid, large-scale manufacturing with consistent quality, lowering overall production costs. Antibodies require biological expression systems, making them more expensive to produce and less adaptable to mass production, limiting scalability in commercial applications.
Future Prospects in Biological Engineering
Aptamers offer significant future potential in biological engineering due to their customizable synthesis, high specificity, and stability under diverse conditions, enabling innovative therapeutic and diagnostic applications. Unlike antibodies, aptamers can be rapidly engineered for targeted drug delivery, biosensing, and molecular imaging, enhancing precision medicine strategies. Continued advancements in SELEX technology and chemical modifications are expected to expand aptamer utility, challenging traditional antibody-based methods in next-generation biological systems.
SELEX (Systematic Evolution of Ligands by EXponential enrichment)
SELEX technology enables the rapid, cost-effective selection of highly specific aptamers as synthetic alternatives to antibodies for targeted molecular recognition.
Affinity maturation
Aptamers undergo in vitro affinity maturation through iterative selection techniques like SELEX, enabling rapid enhancement of binding specificity and affinity compared to the natural affinity maturation process of antibodies.
Monoclonal antibodies
Monoclonal antibodies provide highly specific antigen targeting for diagnostics and therapeutics, while aptamers offer synthetic, cost-effective, and easily modifiable alternatives with comparable affinity and stability.
Oligonucleotide therapeutics
Aptamers, as synthetic oligonucleotide therapeutics, offer higher specificity, lower immunogenicity, and improved stability compared to traditional protein-based antibodies in targeted drug delivery and diagnostic applications.
Epitope specificity
Aptamers offer higher epitope specificity than antibodies due to their synthetic selection process enabling precise binding to unique target sites.
Riboswitches
Riboswitches offer RNA-based regulatory elements that, unlike antibodies, enable aptamers to directly modulate gene expression through conformational changes upon small molecule binding.
Immunogenicity
Aptamers exhibit lower immunogenicity compared to antibodies, making them preferable for therapeutic applications where minimizing immune response is critical.
Binding kinetics
Aptamers exhibit faster association rates and higher binding specificity compared to antibodies, enabling more precise and rapid target recognition in diagnostic and therapeutic applications.
Structural mimicry
Aptamers exhibit superior structural mimicry to antibodies by folding into unique three-dimensional shapes that precisely bind target molecules with high affinity and specificity, enabling versatile applications in diagnostics and therapeutics.
Conjugation chemistry
Aptamers offer versatile conjugation chemistry through site-specific modifications using amine, thiol, or click chemistry, providing more controlled and stable conjugates compared to traditional antibody conjugation methods reliant on lysine or cysteine residues.
Aptamers vs Antibodies Infographic
 njnir.com
njnir.com