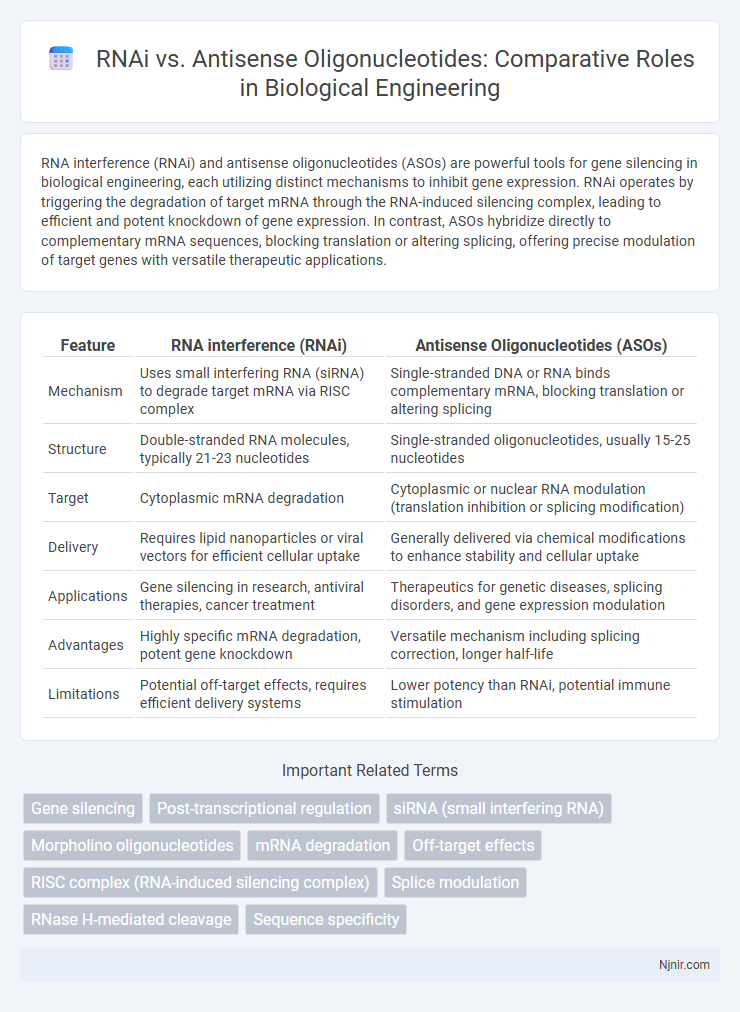
RNA interference (RNAi) and antisense oligonucleotides (ASOs) are powerful tools for gene silencing in biological engineering, each utilizing distinct mechanisms to inhibit gene expression. RNAi operates by triggering the degradation of target mRNA through the RNA-induced silencing complex, leading to efficient and potent knockdown of gene expression. In contrast, ASOs hybridize directly to complementary mRNA sequences, blocking translation or altering splicing, offering precise modulation of target genes with versatile therapeutic applications.
Table of Comparison
| Feature | RNA interference (RNAi) | Antisense Oligonucleotides (ASOs) |
|---|---|---|
| Mechanism | Uses small interfering RNA (siRNA) to degrade target mRNA via RISC complex | Single-stranded DNA or RNA binds complementary mRNA, blocking translation or altering splicing |
| Structure | Double-stranded RNA molecules, typically 21-23 nucleotides | Single-stranded oligonucleotides, usually 15-25 nucleotides |
| Target | Cytoplasmic mRNA degradation | Cytoplasmic or nuclear RNA modulation (translation inhibition or splicing modification) |
| Delivery | Requires lipid nanoparticles or viral vectors for efficient cellular uptake | Generally delivered via chemical modifications to enhance stability and cellular uptake |
| Applications | Gene silencing in research, antiviral therapies, cancer treatment | Therapeutics for genetic diseases, splicing disorders, and gene expression modulation |
| Advantages | Highly specific mRNA degradation, potent gene knockdown | Versatile mechanism including splicing correction, longer half-life |
| Limitations | Potential off-target effects, requires efficient delivery systems | Lower potency than RNAi, potential immune stimulation |
Introduction to Gene Silencing Technologies
RNA interference (RNAi) and antisense oligonucleotides (ASOs) are targeted gene silencing technologies that regulate gene expression by degrading or blocking specific mRNA transcripts. RNAi utilizes small interfering RNAs (siRNAs) or microRNAs (miRNAs) to trigger the RNA-induced silencing complex (RISC), leading to mRNA cleavage and reduced protein synthesis. ASOs are short, synthetic nucleic acid strands that bind complementary mRNA sequences, preventing translation or promoting RNase H-mediated degradation, offering precise therapeutic modulation of disease-related genes.
RNAi: Mechanism and Biological Applications
RNA interference (RNAi) involves small interfering RNA (siRNA) molecules that guide the RNA-induced silencing complex (RISC) to degrade complementary mRNA, effectively silencing gene expression at the post-transcriptional level. This mechanism enables precise regulation of specific genes, making RNAi a powerful tool for studying gene function and developing therapeutic interventions for diseases such as cancer, viral infections, and genetic disorders. RNAi's ability to induce sequence-specific mRNA degradation contrasts with antisense oligonucleotides, which bind directly to mRNA to block translation or alter splicing without mRNA cleavage.
Antisense Oligonucleotides: Mechanism and Therapeutic Potential
Antisense oligonucleotides (ASOs) are short, synthetic strands of nucleotides designed to bind specifically to target RNA sequences, modulating gene expression by promoting RNA degradation or blocking translation. This mechanism allows precise downregulation of pathogenic genes involved in genetic disorders, cancers, and viral infections. The therapeutic potential of ASOs is demonstrated by FDA-approved drugs such as nusinersen for spinal muscular atrophy and mipomersen for familial hypercholesterolemia, highlighting their capacity for targeted and customizable gene silencing.
Molecular Differences Between RNAi and Antisense Approaches
RNA interference (RNAi) utilizes small interfering RNA (siRNA) molecules that are incorporated into the RNA-induced silencing complex (RISC) to cleave complementary target mRNA, leading to gene silencing. Antisense oligonucleotides (ASOs) are single-stranded DNA or RNA analogs that bind directly to target mRNA, blocking translation or modulating splicing without requiring RISC involvement. The fundamental molecular difference lies in RNAi's reliance on enzymatic cleavage via RISC, whereas ASOs primarily function through steric hindrance or RNase H-mediated degradation.
Delivery Strategies for RNAi and Antisense Oligonucleotides
Effective delivery strategies for RNAi and antisense oligonucleotides (ASOs) are critical to their therapeutic success, with lipid nanoparticles (LNPs) and conjugation to cell-penetrating peptides (CPPs) being prominent methods for RNAi. ASOs often utilize chemical modifications such as phosphorothioate backbones and 2'-O-methyl or locked nucleic acid (LNA) residues to enhance stability and cellular uptake without additional carriers. Targeted delivery approaches, including ligand-receptor mediated endocytosis using GalNAc conjugation for liver-specific uptake, demonstrate significant advancements in both RNAi and ASO therapies, improving efficacy and reducing off-target effects.
Efficiency and Specificity of Gene Knockdown
RNA interference (RNAi) offers high efficiency in gene knockdown by utilizing endogenous pathways that degrade target mRNA through RISC complex activity, often achieving robust silencing at low concentrations. Antisense oligonucleotides (ASOs) provide precise specificity by binding complementary RNA sequences and modulating splicing or promoting RNase H-mediated degradation, but their efficiency may vary depending on cellular uptake and target accessibility. RNAi generally exhibits stronger knockdown potency, while ASOs excel in targeting nuclear RNA and distinct splice variants, enabling tailored therapeutic interventions.
Off-Target Effects and Safety Profiles
RNA interference (RNAi) and antisense oligonucleotides (ASOs) both modulate gene expression but differ in off-target effects and safety profiles. RNAi mechanisms often engage the RNA-induced silencing complex (RISC), leading to potential off-target mRNA silencing due to partial sequence complementarity, whereas ASOs exhibit sequence-specific binding with off-target risks arising from unintended hybridization or immune stimulation. Safety profiles show RNAi therapies may induce more robust innate immune responses and cytotoxicity compared to ASOs, which generally demonstrate improved chemical modification strategies to enhance stability and reduce immunogenicity.
Clinical Applications and Approved Therapies
RNA interference (RNAi) and antisense oligonucleotides (ASOs) are both nucleic acid-based therapies targeting mRNA to modulate gene expression, with RNAi using small interfering RNAs (siRNAs) and ASOs employing single-stranded DNA or RNA molecules. Clinically, RNAi has led to approved therapies such as patisiran for hereditary transthyretin amyloidosis and givosiran for acute hepatic porphyria, while ASOs include approved drugs like nusinersen for spinal muscular atrophy and inotersen for hereditary transthyretin amyloidosis. Both platforms show promise in treating genetic disorders, but RNAi typically achieves gene silencing via RISC-mediated cleavage, whereas ASOs modulate mRNA through diverse mechanisms including RNase H-mediated degradation and splicing modulation, influencing therapeutic applications.
Challenges in Translational Research
RNA interference (RNAi) and antisense oligonucleotides (ASOs) both face significant challenges in translational research, including delivery efficiency, off-target effects, and immune system activation. RNAi struggles with stable and tissue-specific delivery of small interfering RNAs (siRNAs) across physiological barriers, while ASOs encounter difficulties in achieving sufficient cellular uptake and durability without inducing toxicity. Overcoming these issues requires advances in nanoparticle carriers, chemical modifications, and targeting strategies to enhance clinical efficacy and safety profiles.
Future Perspectives in Gene Silencing Technologies
RNAi and antisense oligonucleotides represent cutting-edge approaches in gene silencing technologies with distinct mechanisms: RNAi leverages the cellular RNA-induced silencing complex for mRNA degradation, while antisense oligonucleotides bind target RNA to modulate splicing or induce cleavage. The future of gene silencing anticipates enhanced delivery systems for both modalities, addressing cellular uptake and off-target effects to improve precision and efficacy in therapeutic applications. Emerging strategies also include combining RNAi and antisense techniques with CRISPR-based tools for highly specific, multi-layered gene regulation in personalized medicine.
Gene silencing
RNAi achieves gene silencing by degrading target mRNA through the RNA-induced silencing complex, while antisense oligonucleotides inhibit gene expression by directly binding to mRNA to block translation or promote RNase H-mediated cleavage.
Post-transcriptional regulation
RNA interference (RNAi) utilizes small interfering RNAs (siRNAs) to trigger mRNA degradation via the RNA-induced silencing complex (RISC), whereas antisense oligonucleotides (ASOs) bind complementary mRNA sequences to modulate splicing, block translation, or induce RNase H-mediated cleavage, both enabling precise post-transcriptional gene regulation.
siRNA (small interfering RNA)
siRNA (small interfering RNA) utilizes the RNAi pathway to specifically target and degrade complementary mRNA sequences, offering higher gene silencing efficiency compared to antisense oligonucleotides which primarily block translation without mRNA degradation.
Morpholino oligonucleotides
Morpholino oligonucleotides, a type of antisense oligonucleotide, offer superior stability and specificity in gene knockdown by sterically blocking mRNA translation, distinguishing them from RNA interference (RNAi) mechanisms that rely on enzymatic mRNA cleavage.
mRNA degradation
RNAi induces mRNA degradation through the RNA-induced silencing complex (RISC), while antisense oligonucleotides trigger RNase H-mediated mRNA cleavage, both effectively reducing target gene expression.
Off-target effects
RNAi typically exhibits higher off-target effects due to the incorporation of siRNAs into the RNA-induced silencing complex (RISC), which can bind imperfectly to multiple mRNA transcripts, whereas antisense oligonucleotides have more sequence-specific binding, resulting in comparatively lower off-target interactions.
RISC complex (RNA-induced silencing complex)
RNA interference (RNAi) utilizes the RNA-induced silencing complex (RISC) to guide sequence-specific degradation of target mRNA, whereas antisense oligonucleotides primarily inhibit gene expression through steric hindrance or RNase H-mediated cleavage without involving RISC.
Splice modulation
RNAi primarily degrades target mRNA to silence gene expression, whereas antisense oligonucleotides modulate RNA splicing by binding pre-mRNA to alter exon inclusion or exclusion.
RNase H-mediated cleavage
RNase H-mediated cleavage primarily occurs in antisense oligonucleotide therapy, where hybridization to target RNA recruits RNase H to degrade the RNA strand, whereas RNAi involves RISC complex-mediated cleavage without RNase H participation.
Sequence specificity
RNAi demonstrates higher sequence specificity than antisense oligonucleotides by utilizing the RNA-induced silencing complex (RISC) to precisely target complementary mRNA sequences for degradation.
RNAi vs Antisense oligonucleotides Infographic
 njnir.com
njnir.com