Bioprinting enables the precise layering of living cells and biomaterials to create complex tissue structures with high spatial resolution, facilitating advances in regenerative medicine and organ fabrication. Microfluidics offers controlled manipulation of fluids at the microscale, allowing detailed simulation of cellular environments and efficient drug screening platforms. Comparing both, bioprinting excels in structural complexity while microfluidics provides dynamic control of microenvironments, making these technologies complementary in biological engineering applications.
Table of Comparison
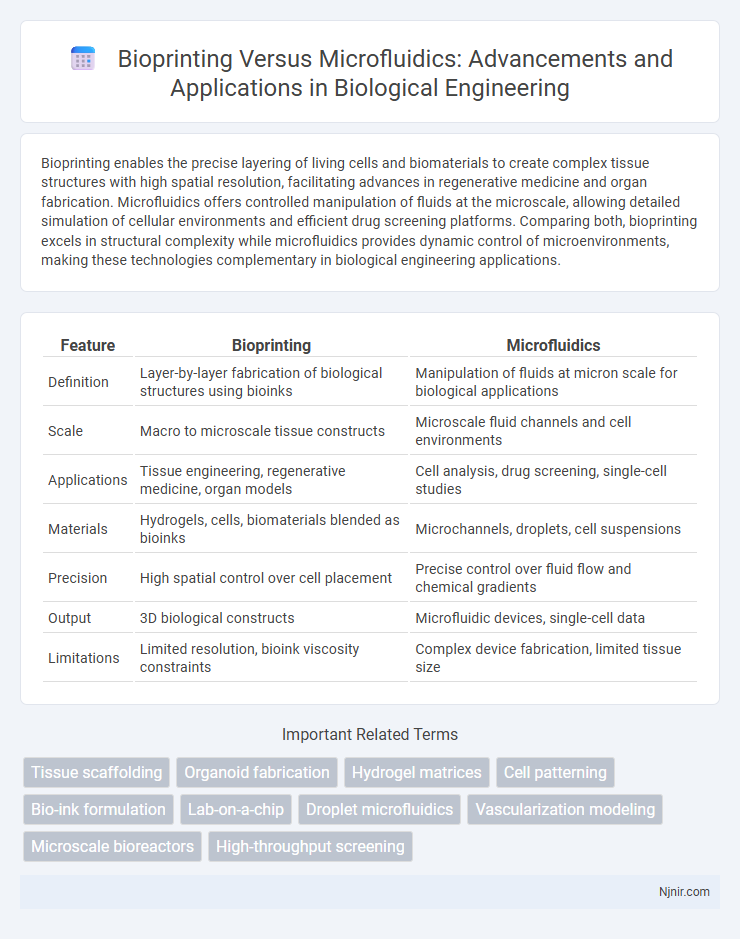
| Feature | Bioprinting | Microfluidics |
|---|---|---|
| Definition | Layer-by-layer fabrication of biological structures using bioinks | Manipulation of fluids at micron scale for biological applications |
| Scale | Macro to microscale tissue constructs | Microscale fluid channels and cell environments |
| Applications | Tissue engineering, regenerative medicine, organ models | Cell analysis, drug screening, single-cell studies |
| Materials | Hydrogels, cells, biomaterials blended as bioinks | Microchannels, droplets, cell suspensions |
| Precision | High spatial control over cell placement | Precise control over fluid flow and chemical gradients |
| Output | 3D biological constructs | Microfluidic devices, single-cell data |
| Limitations | Limited resolution, bioink viscosity constraints | Complex device fabrication, limited tissue size |
Introduction to Bioprinting and Microfluidics
Bioprinting involves layer-by-layer deposition of bioinks composed of living cells and biomaterials to fabricate three-dimensional tissues with precise architecture for regenerative medicine and drug testing. Microfluidics manipulates fluids at the microscale within channels to control cell behavior, perform assays, and create complex tissue environments with high spatial resolution. Both technologies advance tissue engineering by enabling intricate biological structures, but bioprinting emphasizes 3D spatial arrangement while microfluidics focuses on fluid dynamics and microenvironment control.
Fundamental Principles of Bioprinting
Bioprinting relies on layer-by-layer deposition of biomaterials and living cells using techniques such as inkjet, extrusion, and laser-assisted printing to create three-dimensional tissue constructs with high spatial precision. This method emphasizes the controlled placement of bioinks under sterile conditions to preserve cell viability and functionality, enabling the fabrication of complex tissue architectures. Key parameters include bioink rheology, cell density, and print resolution, which collectively influence the structural integrity and biocompatibility of the printed tissues.
Core Concepts in Microfluidics
Microfluidics involves the precise manipulation of fluids at the microliter to picoliter scale within channels typically less than 100 micrometers wide, enabling high-throughput analysis and control over chemical and biological reactions. Core concepts include laminar flow, droplet generation, and mixing within microchannels, which allow for enhanced spatiotemporal resolution and minimal reagent consumption. In contrast to bioprinting, which assembles biological structures layer-by-layer, microfluidics provides dynamic fluid control for applications such as single-cell analysis, organ-on-chip systems, and controlled drug delivery.
Comparative Advantages of Bioprinting
Bioprinting offers superior spatial precision in tissue engineering, enabling the construction of complex, multi-cellular structures with controlled architecture that closely mimics native tissues. It allows for scalable fabrication of customized organ models, enhancing drug testing and regenerative medicine applications. This technology also supports the incorporation of various biomaterials and cell types simultaneously, providing versatility that surpasses the simpler fluid manipulation capabilities of microfluidics.
Unique Strengths of Microfluidic Systems
Microfluidic systems offer unparalleled precision in manipulating fluids at the microscale, enabling controlled chemical gradients and high-throughput screening essential for complex biological assays. Their ability to integrate multiple laboratory functions on a single chip reduces sample volume and increases efficiency, making them ideal for organ-on-a-chip models and real-time cellular analysis. Unlike bioprinting, microfluidics excels in dynamic fluid control and rapid prototyping of microenvironments critical for personalized medicine and drug testing.
Applications in Tissue Engineering
Bioprinting enables precise spatial deposition of cells and biomaterials, facilitating the creation of complex tissue constructs with vascular networks for regenerative medicine and organ replacement. Microfluidics offers controlled microenvironments for cell culture, enabling high-throughput drug screening and the development of organ-on-a-chip models that mimic physiological conditions. Both technologies complement each other, where bioprinting advances the structural complexity of engineered tissues while microfluidics enhances functional analysis and microenvironmental control.
Role in Drug Discovery and Testing
Bioprinting enables the fabrication of complex, multicellular tissue models that closely mimic human physiology, providing accurate platforms for drug screening and toxicity testing. Microfluidics offers precise control over cellular microenvironments and fluid flow, facilitating high-throughput drug assays and real-time monitoring of cellular responses. Integrating bioprinting with microfluidic systems enhances drug discovery by combining structural complexity with dynamic experimental manipulation, improving predictive accuracy for drug efficacy and safety.
Scalability and Throughput Considerations
Bioprinting enables scalable tissue fabrication through layer-by-layer deposition, supporting complex 3D structures but often limited by slower printing speeds and lower throughput. Microfluidics offers high-throughput processing with precise control over microscale fluid dynamics, ideal for generating uniform cell cultures and biochemical gradients but faces challenges in scaling up to larger tissue constructs. Combining bioprinting and microfluidic technologies can potentially overcome throughput limitations while maintaining scalability for advanced tissue engineering applications.
Technological Challenges and Limitations
Bioprinting faces technological challenges such as limited resolution, difficulties in replicating vascular networks, and bioink material constraints affecting cell viability and structural integrity. Microfluidics encounters limitations including channel clogging, precise control over fluid dynamics at microscale, and integration with biological systems for real-time monitoring. Both technologies struggle with scalability and reproducibility, impacting their application in complex tissue engineering and personalized medicine.
Future Trends and Integration Potential
Emerging trends in bioprinting emphasize the precise fabrication of complex tissue structures using advanced biomaterials, while microfluidics continues to enhance cell behavior control and high-throughput screening capabilities at microscale. Integration of bioprinting and microfluidics promises the development of dynamic organ-on-chip systems and vascularized tissue constructs, leveraging the scalability of 3D bioprinting with the fluid manipulation accuracy of microchannels. Future research prioritizes combining these technologies to improve tissue engineering outcomes, personalized medicine applications, and real-time biological analysis.
Tissue scaffolding
Bioprinting enables precise layering of biomaterials to create complex tissue scaffolds, while microfluidics offers fine control of cellular microenvironments for enhanced scaffold perfusion and nutrient delivery.
Organoid fabrication
Bioprinting enables precise spatial arrangement of multiple cell types in organoid fabrication, while microfluidics offers controlled microenvironment and nutrient flow, enhancing organoid growth and maturation.
Hydrogel matrices
Hydrogel matrices in bioprinting enable precise 3D cell placement and structural mimicry, while in microfluidics they facilitate controlled microenvironmental conditions for cell encapsulation and nutrient diffusion.
Cell patterning
Bioprinting enables precise 3D cell patterning by depositing living cells layer-by-layer, while microfluidics controls cell arrangement through fluid dynamics in microchannels for high-throughput and spatially defined cell culture.
Bio-ink formulation
Bio-ink formulation in bioprinting emphasizes cell viability and scaffold integrity while microfluidics optimizes precise control over bio-ink mixing and gradient formation for enhanced tissue engineering applications.
Lab-on-a-chip
Lab-on-a-chip technology integrates microfluidics for precise manipulation of fluids at the microscale, whereas bioprinting enables the layer-by-layer fabrication of complex biological tissues, with lab-on-a-chip systems offering enhanced efficiency and scalability for high-throughput biochemical assays.
Droplet microfluidics
Droplet microfluidics enables precise manipulation of individual picoliter droplets, offering higher throughput and control compared to traditional bioprinting methods in tissue engineering applications.
Vascularization modeling
Bioprinting enables precise 3D vascularization modeling by depositing biomaterials layer-by-layer to create complex blood vessel architectures, whereas microfluidics provides dynamic control of fluid flow within micro-scale channels for simulating functional vascular networks.
Microscale bioreactors
Microscale bioreactors in bioprinting enable precise cell patterning and tissue fabrication, while microfluidics offers controlled fluid dynamics for enhanced nutrient delivery and real-time monitoring in tissue engineering.
High-throughput screening
Bioprinting enables precise spatial organization of cells for high-throughput screening of complex tissue models, while microfluidics offers rapid, scalable manipulation of microenvironments for efficient drug screening and cellular analysis.
Bioprinting vs Microfluidics Infographic
 njnir.com
njnir.com