Recombinant proteins are engineered through genetic modification to be produced in host cells, offering high purity and scalability compared to native proteins extracted directly from organisms. These proteins often exhibit enhanced stability and customizability, enabling targeted therapeutic and industrial applications. However, native proteins maintain natural post-translational modifications crucial for specific biological functions that recombinant counterparts may lack.
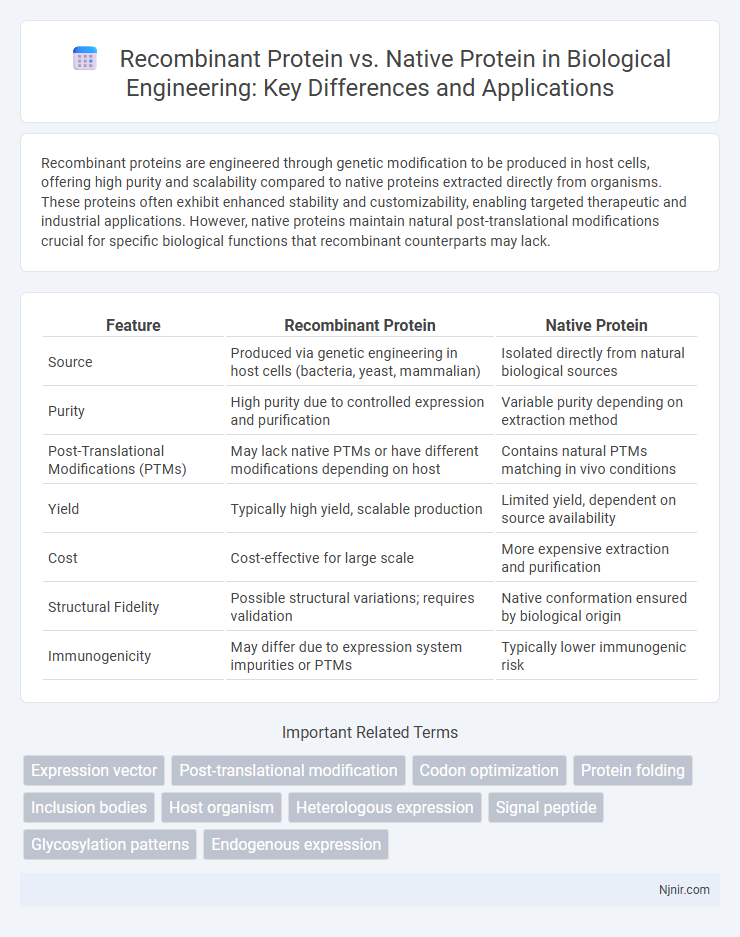
Table of Comparison
| Feature | Recombinant Protein | Native Protein |
|---|---|---|
| Source | Produced via genetic engineering in host cells (bacteria, yeast, mammalian) | Isolated directly from natural biological sources |
| Purity | High purity due to controlled expression and purification | Variable purity depending on extraction method |
| Post-Translational Modifications (PTMs) | May lack native PTMs or have different modifications depending on host | Contains natural PTMs matching in vivo conditions |
| Yield | Typically high yield, scalable production | Limited yield, dependent on source availability |
| Cost | Cost-effective for large scale | More expensive extraction and purification |
| Structural Fidelity | Possible structural variations; requires validation | Native conformation ensured by biological origin |
| Immunogenicity | May differ due to expression system impurities or PTMs | Typically lower immunogenic risk |
Introduction to Recombinant and Native Proteins
Recombinant proteins are engineered by inserting specific genes into host cells, enabling high-yield production of target proteins identical in sequence to native proteins naturally expressed in organisms. Native proteins are those isolated directly from natural biological sources, retaining their original post-translational modifications and three-dimensional structures crucial for biological activity. Understanding differences in expression systems, folding processes, and modifications is essential for applications in research, therapeutics, and diagnostics.
Structural Differences Between Recombinant and Native Proteins
Recombinant proteins often exhibit structural differences from native proteins due to variations in post-translational modifications such as glycosylation patterns, folding processes, and disulfide bond formation, which are influenced by the host expression system used. Native proteins typically possess precise folding and biologically relevant modifications optimized by their original cellular environment, ensuring full functional activity and stability. These structural discrepancies can impact the recombinant protein's biological activity, stability, and immunogenicity, affecting their application in therapeutics and research.
Expression Systems for Recombinant Proteins
Expression systems for recombinant proteins include bacterial, yeast, insect, and mammalian cells, each offering unique advantages for protein yield, folding, and post-translational modifications. Bacterial systems like Escherichia coli provide rapid growth and high expression levels but often lack proper glycosylation, whereas mammalian systems such as CHO cells ensure authentic post-translational modifications essential for native protein function. Selection of an appropriate expression system is crucial to replicate native protein characteristics and enhance therapeutic efficacy and structural fidelity.
Isolation and Purification Processes
Recombinant proteins are typically isolated from host cells such as E. coli, yeast, or mammalian cells, requiring cell lysis and affinity chromatography steps tailored to tagged sequences like His-tags or FLAG-tags, enabling high purity and yield. Native proteins are purified directly from biological tissues or fluids, often involving complex protocols including differential centrifugation, precipitation, and multiple chromatographic techniques to maintain native conformation and activity. Purification of recombinant proteins benefits from genetic engineering for selective binding, whereas native protein isolation demands careful optimization to preserve functional integrity amid diverse cellular components.
Functional Characteristics and Bioactivity Comparison
Recombinant proteins often exhibit greater purity and consistency compared to native proteins, which can contain heterogeneous isoforms and post-translational modifications affecting their functional characteristics. Functional activity of recombinant proteins depends on the expression system used, with eukaryotic systems better replicating native post-translational modifications crucial for bioactivity. Bioactivity assays typically reveal that recombinant proteins may display altered binding affinities or enzymatic activities due to differences in folding or glycosylation patterns compared to their native counterparts.
Post-Translational Modifications: Variations and Implications
Recombinant proteins often exhibit differences in post-translational modifications (PTMs) compared to their native protein counterparts due to variations in host expression systems, such as bacterial, yeast, or mammalian cells. These differences in glycosylation, phosphorylation, and folding can impact protein function, stability, and immunogenicity, necessitating careful selection of expression systems for therapeutic and research applications. Understanding PTM variations is crucial for ensuring recombinant proteins accurately mimic native protein activities and achieve desired biological outcomes.
Yield, Scalability, and Production Efficiency
Recombinant protein production offers higher yield and improved scalability compared to native protein extraction, enabling cost-effective large-scale manufacturing. Native proteins are limited by source availability and complex purification processes, resulting in lower production efficiency. Advanced biotechnological methods in recombinant protein synthesis enhance batch consistency and reduce downstream processing time, optimizing overall production efficiency.
Applications in Biopharmaceuticals and Research
Recombinant proteins, produced through genetic engineering in host cells such as E. coli or CHO cells, offer consistent quality and scalability crucial for biopharmaceuticals like monoclonal antibodies and vaccines. Native proteins, isolated from natural sources, retain authentic post-translational modifications essential for studying biological functions and interactions in research applications. The ability to produce recombinant proteins with specific modifications enhances drug development, whereas native proteins provide baseline data for validating therapeutic targets and understanding disease mechanisms.
Analytical Methods for Protein Characterization
Analytical methods for characterizing recombinant and native proteins include mass spectrometry, circular dichroism, and X-ray crystallography to determine molecular weight, secondary and tertiary structures. Techniques like SDS-PAGE and Western blotting help assess purity and immunoreactivity, while chromatography methods such as HPLC and size-exclusion chromatography evaluate homogeneity and aggregation states. Post-translational modifications in recombinant proteins are often analyzed using peptide mapping and glycosylation profiling to compare with native protein counterparts.
Challenges and Future Perspectives in Protein Engineering
Recombinant proteins often face challenges such as improper folding, post-translational modifications, and reduced biological activity compared to native proteins, which can limit their therapeutic efficacy and stability. Advances in protein engineering, including site-directed mutagenesis and machine learning-guided design, aim to overcome these limitations by enhancing folding accuracy and functional expression in heterologous systems. Future perspectives emphasize developing more efficient expression platforms and optimizing protein conformational fidelity to bridge the gap between recombinant and native protein functionalities for biomedical and industrial applications.
Expression vector
Recombinant proteins are produced using engineered expression vectors that enable high-yield and customizable protein expression, whereas native proteins are naturally synthesized without vector intervention.
Post-translational modification
Recombinant proteins often exhibit altered post-translational modifications compared to native proteins due to differences in host cell machinery, impacting their structure, function, and therapeutic efficacy.
Codon optimization
Codon optimization enhances recombinant protein expression by adapting gene sequences to host-specific tRNA abundance, improving translation efficiency compared to native protein production.
Protein folding
Recombinant proteins often exhibit altered protein folding compared to native proteins due to differences in expression systems, post-translational modifications, and cellular environments affecting their structural conformation and functionality.
Inclusion bodies
Recombinant proteins often form insoluble inclusion bodies during overexpression in host cells, whereas native proteins typically maintain proper folding and solubility in their natural cellular environment.
Host organism
Recombinant proteins are produced in engineered host organisms such as bacteria, yeast, or mammalian cells, whereas native proteins are isolated directly from their original biological sources.
Heterologous expression
Heterologous expression enables large-scale production of recombinant proteins in non-native host systems, often resulting in differ ent post-translational modifications compared to native proteins.
Signal peptide
Recombinant proteins often require engineered signal peptides for efficient secretion, whereas native proteins utilize their inherent signal peptides optimized for cellular export.
Glycosylation patterns
Recombinant proteins often exhibit altered glycosylation patterns compared to native proteins due to differences in host cell machinery, impacting their stability, activity, and immunogenicity.
Endogenous expression
Recombinant proteins often exhibit altered post-translational modifications compared to native proteins due to differences in endogenous expression systems, impacting their functional fidelity.
Recombinant protein vs Native protein Infographic
 njnir.com
njnir.com