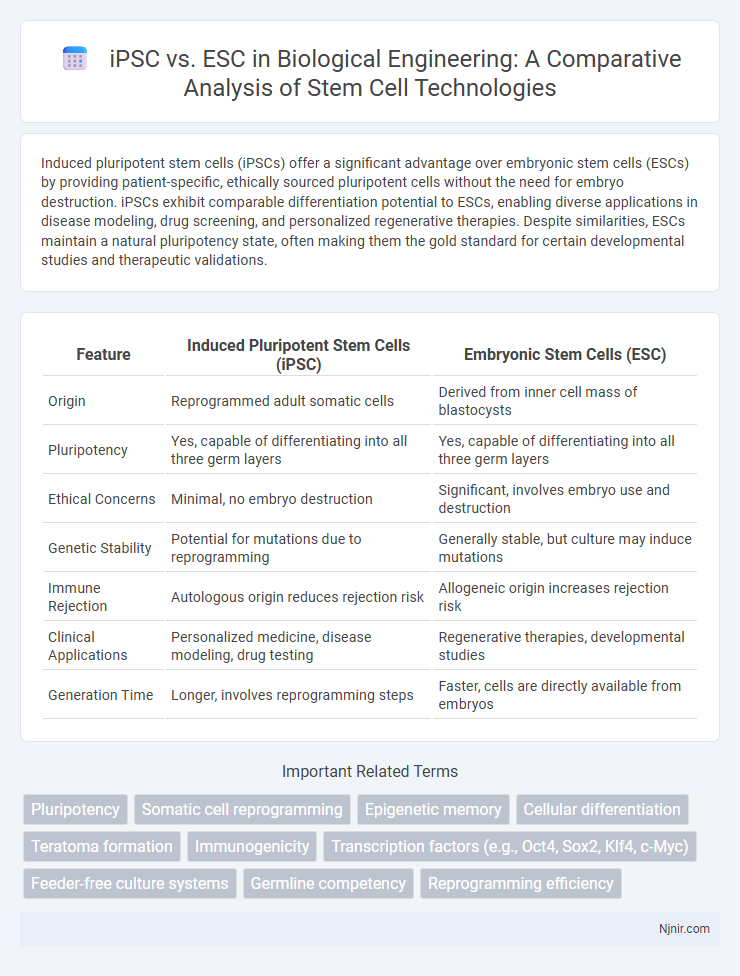
Induced pluripotent stem cells (iPSCs) offer a significant advantage over embryonic stem cells (ESCs) by providing patient-specific, ethically sourced pluripotent cells without the need for embryo destruction. iPSCs exhibit comparable differentiation potential to ESCs, enabling diverse applications in disease modeling, drug screening, and personalized regenerative therapies. Despite similarities, ESCs maintain a natural pluripotency state, often making them the gold standard for certain developmental studies and therapeutic validations.
Table of Comparison
| Feature | Induced Pluripotent Stem Cells (iPSC) | Embryonic Stem Cells (ESC) |
|---|---|---|
| Origin | Reprogrammed adult somatic cells | Derived from inner cell mass of blastocysts |
| Pluripotency | Yes, capable of differentiating into all three germ layers | Yes, capable of differentiating into all three germ layers |
| Ethical Concerns | Minimal, no embryo destruction | Significant, involves embryo use and destruction |
| Genetic Stability | Potential for mutations due to reprogramming | Generally stable, but culture may induce mutations |
| Immune Rejection | Autologous origin reduces rejection risk | Allogeneic origin increases rejection risk |
| Clinical Applications | Personalized medicine, disease modeling, drug testing | Regenerative therapies, developmental studies |
| Generation Time | Longer, involves reprogramming steps | Faster, cells are directly available from embryos |
Introduction to Pluripotent Stem Cells
Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) are both types of pluripotent stem cells capable of differentiating into any cell type in the human body. ESCs are derived from the inner cell mass of blastocysts during early embryonic development, offering a natural source of pluripotency. iPSCs are generated by reprogramming adult somatic cells through the introduction of specific transcription factors, providing an ethical and patient-specific alternative for regenerative medicine.
Defining iPSCs and ESCs
Induced pluripotent stem cells (iPSCs) are adult somatic cells reprogrammed to an embryonic-like pluripotent state through the introduction of specific transcription factors. Embryonic stem cells (ESCs) are derived directly from the inner cell mass of a blastocyst-stage embryo and possess inherent pluripotency. Both iPSCs and ESCs can differentiate into any cell type, but their origins and derivation methods distinguish them in regenerative medicine research.
Source and Derivation Methods
Induced pluripotent stem cells (iPSCs) are derived by reprogramming somatic cells, such as skin fibroblasts or blood cells, through the introduction of specific transcription factors like OCT4, SOX2, KLF4, and c-MYC. Embryonic stem cells (ESCs) originate from the inner cell mass of blastocyst-stage embryos, isolated through in vitro fertilization (IVF) protocols. The iPSC derivation bypasses ethical concerns associated with embryo use, offering a patient-specific, autologous cell source, whereas ESCs provide a naturally pluripotent cell population with robust differentiation capacity.
Genetic and Epigenetic Profiles
Induced pluripotent stem cells (iPSCs) exhibit genetic stability similar to embryonic stem cells (ESCs), but iPSCs often retain epigenetic memory from their somatic cell origins, influencing differentiation potential. ESCs maintain a more uniform and naive epigenetic landscape, characterized by open chromatin states and specific DNA methylation patterns that support pluripotency. Comparative analyses reveal that while both cell types share core pluripotency gene expression, distinct epigenetic variations impact their developmental trajectories and therapeutic applications.
Ethical Considerations
Induced pluripotent stem cells (iPSCs) bypass ethical concerns associated with embryonic stem cells (ESCs) as they are reprogrammed from adult somatic cells without destroying embryos. ESC research raises moral debates due to embryo use and potential destruction during extraction, leading to strict regulations in many countries. iPSCs offer a promising alternative, enabling pluripotent stem cell research and therapies while minimizing ethical dilemmas linked to embryonic sources.
Immunogenicity and Compatibility
Induced pluripotent stem cells (iPSCs) exhibit lower immunogenicity compared to embryonic stem cells (ESCs) due to their derivation from the patient's own somatic cells, enhancing compatibility for personalized therapies. ESCs, originating from unrelated donors, often require immunosuppressive treatment to prevent rejection, posing significant challenges in transplantation. Recent studies highlight that iPSCs maintain immune tolerance and reduce risks associated with graft-versus-host disease, making them superior candidates for regenerative medicine applications.
Differentiation Potential
Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) both exhibit pluripotency, enabling differentiation into cell types from all three germ layers: ectoderm, mesoderm, and endoderm. ESCs are naturally pluripotent cells derived from the inner cell mass of blastocysts, demonstrating robust and consistent differentiation potential across various cell lineages. iPSCs, reprogrammed from somatic cells, closely mimic ESCs in differentiation capacity but may show variability influenced by donor cell origin and reprogramming methods.
Applications in Disease Modeling
iPSC (induced pluripotent stem cells) and ESC (embryonic stem cells) both serve as vital tools in disease modeling by enabling the study of genetic disorders and drug response in patient-specific cell types. iPSCs are particularly advantageous due to their ability to be generated from adult somatic cells, allowing personalized disease models without ethical concerns associated with ESCs derived from embryos. ESCs offer consistent pluripotency and stable lines, making them valuable for modeling early developmental diseases and high-throughput drug screening.
Therapeutic and Regenerative Uses
Induced pluripotent stem cells (iPSCs) offer a personalized therapeutic advantage by enabling patient-specific cell generation, reducing immune rejection risks compared to embryonic stem cells (ESCs). ESCs, derived from early embryos, possess a robust differentiation potential and have been extensively studied for regenerative medicine applications in neurodegenerative diseases, cardiac repair, and diabetes. Both iPSCs and ESCs contribute significantly to tissue engineering and regenerative therapies, but iPSCs provide ethical advantages and feasibility for autologous transplantation.
Future Prospects and Challenges
Induced pluripotent stem cells (iPSCs) offer promising future prospects due to their potential for personalized medicine and reduced ethical concerns compared to embryonic stem cells (ESCs). Key challenges for iPSCs include genetic instability, potential tumorigenicity, and scalability for clinical applications, which currently limit their widespread therapeutic use. Continued research aims to overcome these obstacles by improving reprogramming techniques and ensuring the safety and efficacy of iPSC-derived therapies.
Pluripotency
Induced pluripotent stem cells (iPSCs) exhibit comparable pluripotency to embryonic stem cells (ESCs), enabling differentiation into all three germ layers while offering advantages in patient-specific therapy and ethical considerations.
Somatic cell reprogramming
Somatic cell reprogramming transforms adult cells into induced pluripotent stem cells (iPSCs), bypassing ethical concerns associated with embryonic stem cells (ESCs) derived from early embryos.
Epigenetic memory
Induced pluripotent stem cells (iPSCs) retain epigenetic memory from their somatic cell origin, leading to differences in gene expression and differentiation potential compared to embryonic stem cells (ESCs), which possess a more naive epigenetic state.
Cellular differentiation
Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) both exhibit pluripotency, but iPSCs, derived from somatic cells, offer patient-specific cellular differentiation potential with reduced ethical concerns compared to ESCs, which originate from the inner cell mass of blastocysts.
Teratoma formation
Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) both possess teratoma formation potential, but iPSCs often exhibit variable and sometimes higher teratogenic risk due to epigenetic memory and reprogramming inconsistencies.
Immunogenicity
Induced pluripotent stem cells (iPSCs) exhibit lower immunogenicity and reduced risk of immune rejection compared to embryonic stem cells (ESCs) due to their autologous origin, enhancing their potential for personalized regenerative therapies.
Transcription factors (e.g., Oct4, Sox2, Klf4, c-Myc)
Induced pluripotent stem cells (iPSCs) are generated by reprogramming somatic cells using key transcription factors Oct4, Sox2, Klf4, and c-Myc, whereas embryonic stem cells (ESCs) naturally express these factors to maintain pluripotency.
Feeder-free culture systems
Feeder-free culture systems enhance the scalability and clinical applicability of both induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) by providing xeno-free, defined media conditions that reduce variability and contamination risks.
Germline competency
Induced pluripotent stem cells (iPSCs) exhibit lower germline competency compared to embryonic stem cells (ESCs), which maintain robust ability to contribute to germline transmission in vivo.
Reprogramming efficiency
Induced pluripotent stem cells (iPSCs) exhibit lower reprogramming efficiency compared to embryonic stem cells (ESCs), typically achieving success rates of 0.01% to 1%, whereas ESCs inherently maintain pluripotency without reprogramming.
iPSC vs ESC Infographic
 njnir.com
njnir.com