Gene drive technology enables the biased inheritance of specific genetic traits through populations, accelerating the spread of targeted genes more rapidly than traditional Mendelian inheritance. Gene knockout involves disabling or removing a specific gene to study its function or create desired phenotypic changes, typically within an organism's genome. While gene knockout provides precise gene function analysis, gene drive offers powerful applications in population control and ecological management by altering gene frequencies across generations.
Table of Comparison
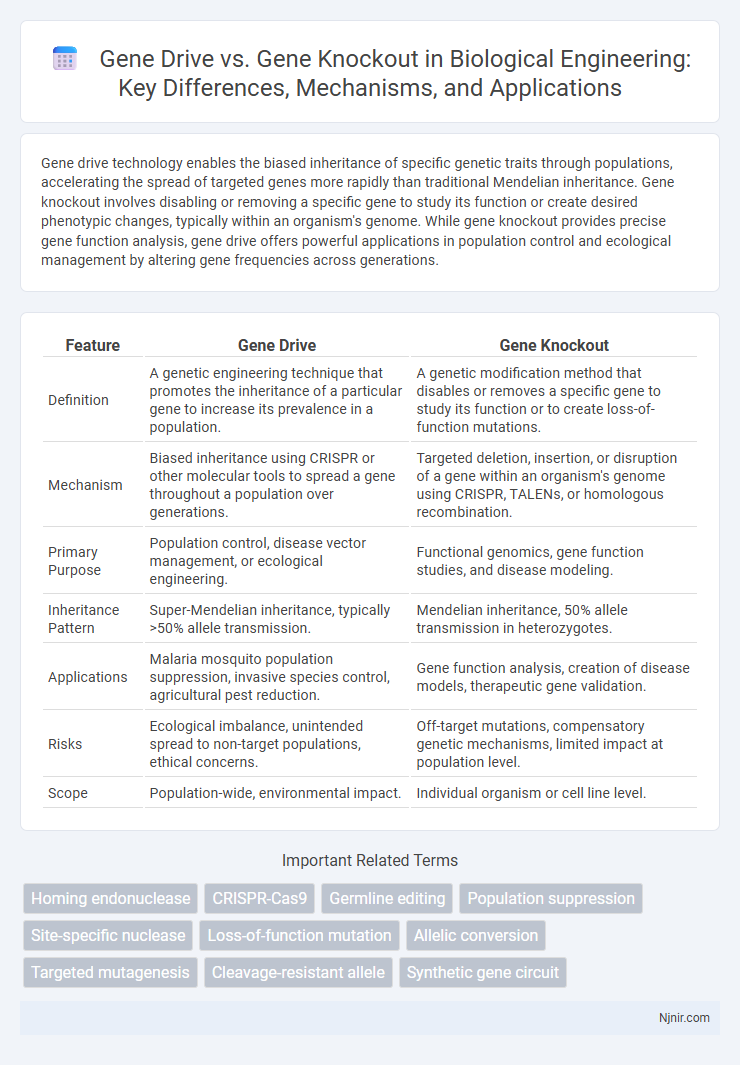
| Feature | Gene Drive | Gene Knockout |
|---|---|---|
| Definition | A genetic engineering technique that promotes the inheritance of a particular gene to increase its prevalence in a population. | A genetic modification method that disables or removes a specific gene to study its function or to create loss-of-function mutations. |
| Mechanism | Biased inheritance using CRISPR or other molecular tools to spread a gene throughout a population over generations. | Targeted deletion, insertion, or disruption of a gene within an organism's genome using CRISPR, TALENs, or homologous recombination. |
| Primary Purpose | Population control, disease vector management, or ecological engineering. | Functional genomics, gene function studies, and disease modeling. |
| Inheritance Pattern | Super-Mendelian inheritance, typically >50% allele transmission. | Mendelian inheritance, 50% allele transmission in heterozygotes. |
| Applications | Malaria mosquito population suppression, invasive species control, agricultural pest reduction. | Gene function analysis, creation of disease models, therapeutic gene validation. |
| Risks | Ecological imbalance, unintended spread to non-target populations, ethical concerns. | Off-target mutations, compensatory genetic mechanisms, limited impact at population level. |
| Scope | Population-wide, environmental impact. | Individual organism or cell line level. |
Introduction to Gene Drive and Gene Knockout
Gene drive is a genetic engineering technology designed to propagate a particular gene throughout a population by biasing inheritance, ensuring a higher-than-Mendelian transmission rate. Gene knockout involves the precise disruption or deletion of a specific gene within an organism's genome to study gene function or create loss-of-function mutations. While gene drive aims to spread traits rapidly across populations, gene knockout focuses on understanding individual gene roles through targeted gene inactivation.
Mechanisms of Gene Drive Systems
Gene drive systems utilize biased inheritance mechanisms such as homing endonuclease genes, CRISPR-Cas9 based cleavage, or Medea elements to propagate specific genetic traits through populations at rates exceeding Mendelian inheritance. These mechanisms involve targeted DNA cleavage and subsequent repair processes like homology-directed repair to copy the gene drive element onto homologous chromosomes, ensuring preferential transmission. In contrast, gene knockout techniques rely on disrupting or deleting specific gene sequences without altering inheritance patterns, typically through non-homologous end joining after targeted DNA breaks.
Principles of Gene Knockout Techniques
Gene knockout techniques involve disrupting or deleting a specific gene to study its function by creating a loss-of-function mutation, often using CRISPR-Cas9, TALENs, or zinc-finger nucleases to introduce double-strand breaks at target loci. Homology-directed repair (HDR) or non-homologous end joining (NHEJ) pathways facilitate gene disruption through precise insertions or frameshift mutations, resulting in inactivated gene expression. This contrasts with gene drive systems, which bias inheritance to spread a genetic alteration rapidly through populations rather than simply knocking out a gene.
Applications in Biological Engineering
Gene drive technology enables the rapid spread of genetic traits through populations, making it effective for controlling vector-borne diseases, invasive species, and pest management in ecological settings. Gene knockout techniques precisely inactivate specific genes to study gene function, develop disease models, and engineer organisms with desired traits in agriculture and medicine. Both tools are pivotal in biological engineering for advancing genetic research, but gene drives offer population-level genetic alterations while knockouts target individual organisms at the genomic level.
Efficiency and Specificity of Genetic Modification
Gene drive technology exhibits higher efficiency in propagating specific genetic traits through populations by biasing inheritance, ensuring nearly all offspring inherit the modified gene, whereas gene knockout typically targets single organisms or cells, limiting spread beyond initial subjects. Specificity in gene knockout relies on precise CRISPR-Cas9 targeting to disrupt or remove a gene, achieving high accuracy but confined to individual genome edits, while gene drives may cause off-target effects due to repeated propagation across generations. Overall, gene drives offer greater potential for population-wide genetic modification with efficiency but require careful design to maintain specificity and minimize unintended consequences.
Ecological and Evolutionary Impacts
Gene drives rapidly propagate genetic traits through populations, potentially disrupting ecosystems by altering species interactions and reducing genetic diversity, which may lead to unintended evolutionary consequences. Gene knockouts target specific genes to study function or eliminate traits but generally have localized ecological effects with slower evolutionary impact compared to gene drives. Both methods require careful assessment of ecological balance and long-term evolutionary risks to avoid irreversible changes in biodiversity.
Ethical and Biosafety Considerations
Gene drive technologies raise significant ethical concerns due to their potential to alter entire populations and ecosystems irreversibly, prompting debates on consent, ecological balance, and long-term impacts. Gene knockout techniques, while more controlled and confined to individual organisms, still pose biosafety risks related to off-target effects and unintended genetic consequences. Both methods require rigorous risk assessment, transparent regulatory frameworks, and ongoing monitoring to ensure responsible use in research and applications.
Challenges and Limitations
Gene drive technology faces challenges including unintended spread to non-target populations and ecological disruptions due to its ability to propagate genetic changes rapidly across species. Gene knockout techniques encounter limitations in off-target effects and incomplete gene disruption, which may lead to inconclusive functional studies or compensation by redundant pathways. Both methods require stringent ethical considerations and robust containment strategies to mitigate risks associated with genetic modifications.
Recent Advances and Case Studies
Recent advances in gene drive technologies have enabled more efficient spread of genetic traits through populations, exemplified by CRISPR-based gene drives in malaria vector mosquitoes significantly reducing Plasmodium transmission. In contrast, gene knockout techniques, utilizing CRISPR-Cas9, have achieved precise disruption of target genes in model organisms like mice and zebrafish, facilitating functional genomic studies and disease modeling. Case studies highlight gene drive applications in controlling invasive species while gene knockouts continue to advance therapeutic research and agricultural improvements.
Future Prospects in Gene Editing Technologies
Gene drive technology offers the potential to rapidly propagate specific genetic traits through populations, making it a powerful tool for controlling vector-borne diseases and invasive species, while gene knockout provides targeted gene disruption to study gene function and develop precise treatments for genetic disorders. Advances in CRISPR/Cas systems are expected to enhance the specificity and efficiency of both methods, reducing off-target effects and expanding their applications in agriculture, medicine, and environmental management. The future of gene editing lies in integrating gene drive's population-level impact with gene knockout's precision to address complex biological challenges sustainably.
Homing endonuclease
Homing endonuclease-based gene drives enable biased inheritance by copying themselves into homologous chromosomes, whereas gene knockouts disrupt specific genes without promoting gene spread.
CRISPR-Cas9
CRISPR-Cas9 enables gene drives to propagate desired genetic traits through populations by biased inheritance, whereas gene knockout uses CRISPR-Cas9 to disrupt or disable specific genes within individual organisms.
Germline editing
Gene drive enables the propagation of specific genetic traits through germline editing across populations at an accelerated rate, while gene knockout involves precise germline modifications to inactivate targeted genes without intentionally spreading alterations beyond the individual organism.
Population suppression
Gene drive technology enables efficient propagation of gene knockouts across populations, enhancing population suppression by biasing inheritance to reduce target species' reproductive success.
Site-specific nuclease
Site-specific nucleases enable precise gene drive by promoting biased inheritance through targeted DNA cleavage and repair, while gene knockout utilizes these nucleases to disrupt gene function by introducing mutations at specific genomic sites.
Loss-of-function mutation
Gene drive promotes the rapid spread of loss-of-function mutations throughout a population by biased inheritance, while gene knockout induces targeted loss-of-function mutations within individual organisms via precise genome editing.
Allelic conversion
Gene drive enables biased allelic conversion to propagate specific genetic traits through populations, while gene knockout achieves loss of function by disrupting target alleles without promoting biased inheritance.
Targeted mutagenesis
Gene drive technology propagates specific genetic modifications through populations by biasing inheritance, while gene knockout achieves targeted mutagenesis by disrupting or deleting specific genes to study their function or create desired traits.
Cleavage-resistant allele
Gene drive technology actively spreads cleavage-resistant alleles through populations by biasing inheritance, whereas gene knockout techniques typically result in fixed, non-driving mutations without influencing allele frequency dynamics.
Synthetic gene circuit
Synthetic gene circuits utilize gene drives to propagate targeted genetic changes through populations, whereas gene knockouts precisely disrupt specific genes within individual organisms without population-level inheritance.
Gene drive vs Gene knockout Infographic
 njnir.com
njnir.com