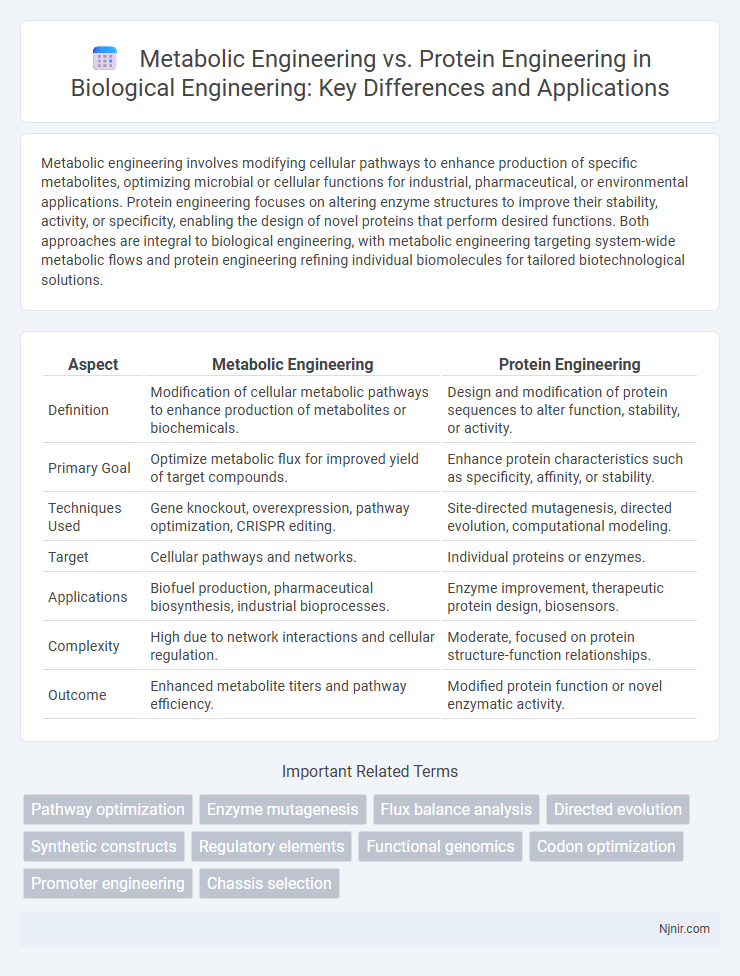
Metabolic engineering involves modifying cellular pathways to enhance production of specific metabolites, optimizing microbial or cellular functions for industrial, pharmaceutical, or environmental applications. Protein engineering focuses on altering enzyme structures to improve their stability, activity, or specificity, enabling the design of novel proteins that perform desired functions. Both approaches are integral to biological engineering, with metabolic engineering targeting system-wide metabolic flows and protein engineering refining individual biomolecules for tailored biotechnological solutions.
Table of Comparison
| Aspect | Metabolic Engineering | Protein Engineering |
|---|---|---|
| Definition | Modification of cellular metabolic pathways to enhance production of metabolites or biochemicals. | Design and modification of protein sequences to alter function, stability, or activity. |
| Primary Goal | Optimize metabolic flux for improved yield of target compounds. | Enhance protein characteristics such as specificity, affinity, or stability. |
| Techniques Used | Gene knockout, overexpression, pathway optimization, CRISPR editing. | Site-directed mutagenesis, directed evolution, computational modeling. |
| Target | Cellular pathways and networks. | Individual proteins or enzymes. |
| Applications | Biofuel production, pharmaceutical biosynthesis, industrial bioprocesses. | Enzyme improvement, therapeutic protein design, biosensors. |
| Complexity | High due to network interactions and cellular regulation. | Moderate, focused on protein structure-function relationships. |
| Outcome | Enhanced metabolite titers and pathway efficiency. | Modified protein function or novel enzymatic activity. |
Introduction to Metabolic and Protein Engineering
Metabolic engineering involves the redirection of cellular pathways to enhance the production of specific metabolites by modifying genes, enzymes, and regulatory mechanisms. Protein engineering focuses on designing and constructing novel proteins or improving existing ones through techniques such as site-directed mutagenesis and directed evolution. Both disciplines integrate molecular biology, genetics, and computational tools to optimize biological function for applications in biotechnology, pharmaceuticals, and biofuels.
Historical Evolution of Metabolic vs Protein Engineering
Metabolic engineering emerged in the late 20th century as a multidisciplinary field focused on optimizing cellular pathways through genetic modifications to enhance production of desired metabolites. Protein engineering developed earlier, originating from studies on enzyme structure and function in the 1960s, evolving with techniques like site-directed mutagenesis to tailor protein properties. Both fields have since advanced with synthetic biology and computational design, yet metabolic engineering emphasizes pathway-level interventions, whereas protein engineering targets individual macromolecule modifications.
Core Principles and Approaches
Metabolic engineering involves the redesign of cellular pathways by modifying genes and enzymes to enhance the production of desired metabolites, relying heavily on systems biology and pathway optimization techniques. Protein engineering focuses on the design and modification of protein structures to improve their functions or create novel activities, utilizing methods such as directed evolution and rational design. Core principles differ in scope, where metabolic engineering addresses entire metabolic networks, while protein engineering targets specific protein molecules for functional enhancement.
Tools and Technologies Involved
Metabolic engineering primarily utilizes genome editing tools such as CRISPR-Cas9, TALENs, and metabolic flux analysis software to modify and optimize cellular pathways for enhanced production of desired metabolites. Protein engineering leverages technologies like directed evolution, site-directed mutagenesis, and computational modeling tools including Rosetta and AlphaFold to design proteins with improved functionality, stability, or specificity. Both fields integrate high-throughput screening methods and advanced bioinformatics platforms to accelerate the design-build-test-learn cycle in synthetic biology applications.
Applications in Biotechnology
Metabolic engineering enhances microbial production of biofuels, pharmaceuticals, and specialty chemicals by optimizing cellular pathways for improved yield and efficiency. Protein engineering enables the design of enzymes with enhanced stability, specificity, and activity, facilitating industrial biocatalysis, therapeutic protein development, and biosensor innovation. Both fields drive advancements in synthetic biology, enabling sustainable production and novel biotechnological applications.
Advantages and Limitations
Metabolic engineering offers the advantage of optimizing entire cellular pathways to enhance production of desired metabolites, enabling large-scale biosynthesis of complex compounds. However, it faces limitations such as pathway complexity, regulatory challenges, and unintended metabolic burden on host cells. Protein engineering allows precise modification of enzyme properties, improving catalytic efficiency and substrate specificity, but often requires extensive structural knowledge and can be limited by protein folding and stability constraints.
Case Studies: Real-World Examples
Metabolic engineering showcases success in producing biofuels and pharmaceuticals, exemplified by engineering E. coli to synthesize insulin and artemisinin precursors, highlighting pathway optimization for industrial-scale production. Protein engineering demonstrates advances in enzyme stability and activity, with directed evolution techniques producing variants like T4 DNA polymerase for high-fidelity PCR and enhanced cellulases for biomass degradation. Both fields leverage synthetic biology tools to tailor biological functions, enabling innovations in healthcare, agriculture, and sustainable manufacturing.
Challenges and Future Directions
Metabolic engineering faces challenges such as complex pathway optimization, balancing cellular resources, and unintended metabolic burdens that limit product yield and strain stability. Protein engineering struggles with predicting protein folding and function, as well as achieving high-throughput screening for desired traits. Future directions emphasize integrating machine learning with synthetic biology tools to enhance pathway design and protein modification precision, driving more efficient bioproduction and therapeutic applications.
Synergies and Integration Opportunities
Metabolic engineering and protein engineering intersect through the optimization of cellular pathways and enzyme functionalities, enabling enhanced biosynthesis of target compounds. Integration opportunities arise when protein engineering tailors enzyme specificity and activity, which metabolic engineering leverages to redesign metabolic fluxes for improved yield and efficiency. Synergies between these fields accelerate the development of robust microbial cell factories for pharmaceuticals, biofuels, and specialty chemicals by combining pathway optimization with enzyme performance enhancements.
Conclusion and Outlook
Metabolic engineering optimizes entire cellular pathways to enhance the production of target metabolites, while protein engineering focuses on improving specific enzyme functions for increased catalytic efficiency and stability. Advances in synthetic biology and machine learning are driving innovations in both fields, enabling the design of more robust metabolic networks and tailored proteins with novel functionalities. Future research will likely integrate these approaches to develop highly efficient biocatalysts and sustainable bioprocesses for industrial and medical applications.
Pathway optimization
Metabolic engineering enhances pathway optimization by modifying entire cellular networks to increase product yield, while protein engineering focuses on improving individual enzyme functions within those pathways for greater catalytic efficiency.
Enzyme mutagenesis
Enzyme mutagenesis in metabolic engineering optimizes entire biosynthetic pathways by altering multiple enzymes for improved metabolite production, whereas protein engineering focuses on precise modifications to individual enzymes to enhance their catalytic efficiency, stability, or specificity.
Flux balance analysis
Flux balance analysis quantifies metabolic pathway efficiencies in metabolic engineering by optimizing reaction fluxes, whereas protein engineering focuses on modifying enzyme structures without directly modeling network-wide flux distributions.
Directed evolution
Directed evolution accelerates protein engineering by iteratively selecting protein variants for desired traits, whereas metabolic engineering applies genetic modifications to entire pathways for optimized biochemical production.
Synthetic constructs
Metabolic engineering optimizes synthetic constructs to enhance cellular pathways for improved biochemical production, while protein engineering designs synthetic protein constructs to alter enzyme functions and specificity at the molecular level.
Regulatory elements
Metabolic engineering leverages regulatory elements such as promoters, enhancers, and riboswitches to optimize entire biosynthetic pathways, whereas protein engineering focuses on modifying regulatory motifs within protein domains to alter enzyme activity and regulation.
Functional genomics
Metabolic engineering enhances cellular pathways for improved biochemical production by integrating functional genomics data, while protein engineering optimizes individual enzyme functions using insights from functional genomics to tailor protein activities.
Codon optimization
Codon optimization in metabolic engineering enhances gene expression for improved pathway efficiency, while in protein engineering it specifically tailors codon usage to increase protein yield and functional performance.
Promoter engineering
Promoter engineering in metabolic engineering optimizes gene expression to enhance whole-pathway flux, while in protein engineering it precisely regulates target gene transcription to improve individual protein production and functionality.
Chassis selection
Chassis selection in metabolic engineering prioritizes microorganisms with robust metabolic networks and genetic tractability for enhanced pathway integration, whereas protein engineering emphasizes host systems that optimize protein expression, folding, and post-translational modifications.
Metabolic engineering vs Protein engineering Infographic
 njnir.com
njnir.com