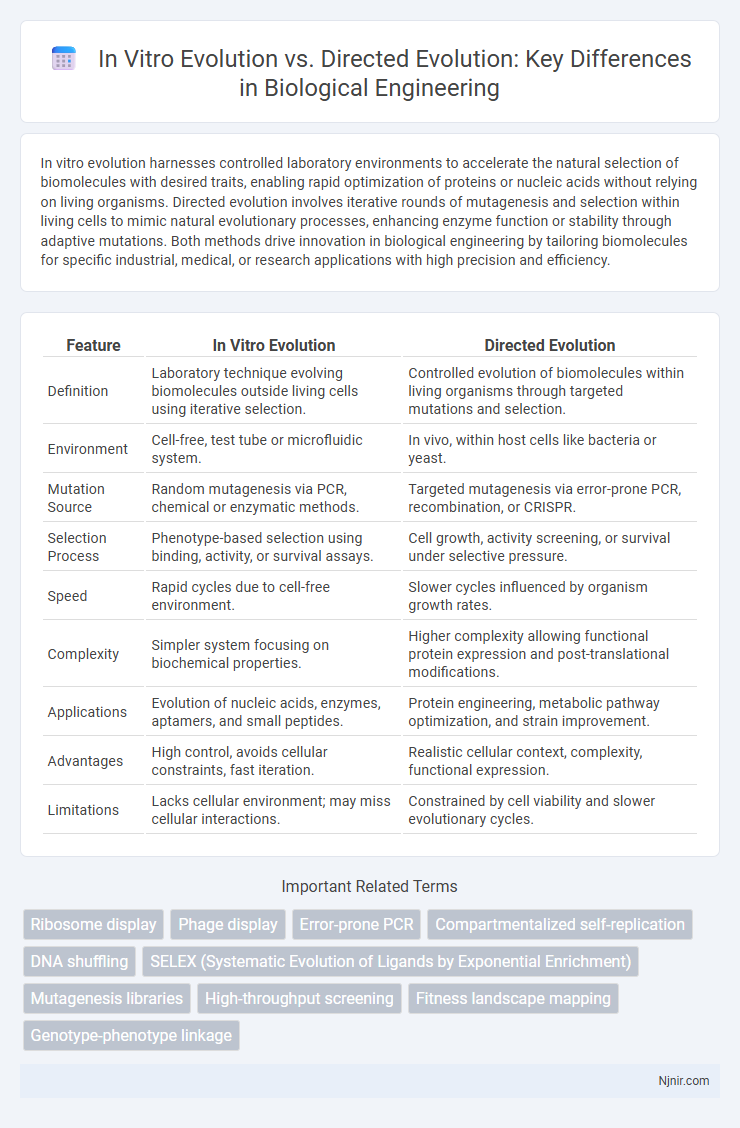
In vitro evolution harnesses controlled laboratory environments to accelerate the natural selection of biomolecules with desired traits, enabling rapid optimization of proteins or nucleic acids without relying on living organisms. Directed evolution involves iterative rounds of mutagenesis and selection within living cells to mimic natural evolutionary processes, enhancing enzyme function or stability through adaptive mutations. Both methods drive innovation in biological engineering by tailoring biomolecules for specific industrial, medical, or research applications with high precision and efficiency.
Table of Comparison
| Feature | In Vitro Evolution | Directed Evolution |
|---|---|---|
| Definition | Laboratory technique evolving biomolecules outside living cells using iterative selection. | Controlled evolution of biomolecules within living organisms through targeted mutations and selection. |
| Environment | Cell-free, test tube or microfluidic system. | In vivo, within host cells like bacteria or yeast. |
| Mutation Source | Random mutagenesis via PCR, chemical or enzymatic methods. | Targeted mutagenesis via error-prone PCR, recombination, or CRISPR. |
| Selection Process | Phenotype-based selection using binding, activity, or survival assays. | Cell growth, activity screening, or survival under selective pressure. |
| Speed | Rapid cycles due to cell-free environment. | Slower cycles influenced by organism growth rates. |
| Complexity | Simpler system focusing on biochemical properties. | Higher complexity allowing functional protein expression and post-translational modifications. |
| Applications | Evolution of nucleic acids, enzymes, aptamers, and small peptides. | Protein engineering, metabolic pathway optimization, and strain improvement. |
| Advantages | High control, avoids cellular constraints, fast iteration. | Realistic cellular context, complexity, functional expression. |
| Limitations | Lacks cellular environment; may miss cellular interactions. | Constrained by cell viability and slower evolutionary cycles. |
Introduction to In Vitro Evolution and Directed Evolution
In vitro evolution utilizes cell-free systems to mimic natural selection processes, enabling rapid identification of biomolecules with desired traits by iteratively selecting and amplifying functional variants. Directed evolution, performed within living cells or organisms, leverages genetic diversification techniques such as error-prone PCR and DNA shuffling combined with high-throughput screening to enhance protein functions. Both approaches accelerate enzyme optimization and drug discovery by harnessing evolutionary principles under controlled laboratory conditions.
Historical Development and Key Milestones
In vitro evolution originated in the 1960s with the advent of techniques like SELEX for nucleic acids, enabling the selection of molecules with desired properties outside living cells. Directed evolution emerged prominently in the 1990s, pioneered by Frances Arnold, who developed iterative rounds of mutagenesis and selection to evolve enzymes with enhanced functions. Key milestones include the first in vitro selection of RNA aptamers and Arnold's Nobel-winning work in 2018 for directed enzyme evolution, marking significant advances in protein engineering and synthetic biology.
Fundamental Principles of In Vitro Evolution
In vitro evolution relies on iterative rounds of mutation and selection conducted entirely outside living organisms to optimize biomolecules under controlled laboratory conditions. Techniques such as ribosome display, mRNA display, and SELEX enable the generation and screening of vast libraries of nucleic acids or proteins based on binding affinity and catalytic activity. This contrasts with directed evolution, which often involves mutagenesis and selection within living cells, emphasizing the fundamental principle of decoupling evolutionary processes from cellular contexts in in vitro evolution for precise molecular engineering.
Core Concepts in Directed Evolution Methods
Directed evolution harnesses iterative cycles of mutation, selection, and amplification to optimize biomolecules for desired traits. Core methods include error-prone PCR for inducing genetic diversity, DNA shuffling to recombine beneficial mutations, and high-throughput screening or selection to identify improved variants. This approach mimics natural evolution in the laboratory, enabling rapid refinement of enzymes, antibodies, and other proteins for industrial and therapeutic applications.
Comparative Analysis: In Vitro vs Directed Evolution
In vitro evolution utilizes cell-free systems to rapidly generate genetic diversity and select biomolecules with desired traits, offering precise control over experimental conditions and faster iteration cycles compared to directed evolution. Directed evolution involves iterative rounds of mutagenesis and selection within living organisms, enabling the incorporation of natural cellular context and complexity that can enhance protein folding and functionality. Both methods have distinct advantages: in vitro evolution excels in speed and customizable selection pressures, while directed evolution benefits from physiological relevance and scalability in producing functional biomolecules.
Technological Tools and Methodological Approaches
In vitro evolution employs high-throughput screening and selection techniques such as phage display and ribosome display to generate and identify biomolecules with desired traits from vast synthetic libraries. Directed evolution utilizes iterative rounds of random mutagenesis, DNA shuffling, and recombination coupled with functional assays in living cells to progressively enhance specific protein functions. Both methodologies leverage advanced molecular biology tools, but in vitro evolution focuses on cell-free systems enabling rapid diversification, whereas directed evolution is grounded in cellular context to better mimic natural selection environments.
Applications in Protein Engineering and Enzyme Design
In vitro evolution employs iterative cycles of mutation and selection to generate proteins with enhanced or novel functions, enabling the rapid optimization of enzyme activity, stability, and substrate specificity. Directed evolution mimics natural selection within laboratory settings, using techniques such as error-prone PCR and DNA shuffling to create diverse protein libraries for identifying variants with improved catalytic efficiency or resistance to harsh conditions. These methods have revolutionized protein engineering by facilitating the design of enzymes for industrial biocatalysis, pharmaceutical development, and environmental applications.
Advantages and Limitations: A Semantic Overview
In vitro evolution enables rapid screening of vast genetic libraries under controlled conditions, optimizing proteins with high specificity and diversity. Directed evolution mimics natural selection cycles in a laboratory, offering practical adaptation of enzymes or organisms to desired traits without requiring detailed structural knowledge. However, in vitro evolution may face limitations in replicating cellular environments, whereas directed evolution is time-consuming and often constrained by the host organism's viability.
Case Studies in Biological Engineering
In vitro evolution and directed evolution are pivotal techniques in biological engineering, enabling the development of proteins and enzymes with enhanced or novel functions. Case studies highlight the engineering of enzymes like cytochrome P450 through directed evolution to improve drug metabolism, while in vitro evolution has been used to evolve nucleic acids with high affinity for therapeutic targets. These methodologies demonstrate superior adaptability for tailoring biomolecules in pharmaceuticals, industrial catalysts, and biosensors, emphasizing their critical role in advancing synthetic biology applications.
Future Perspectives and Emerging Trends
In vitro evolution leverages cell-free systems to accelerate the generation of diverse biomolecule libraries with precise control over selection pressures, enabling the discovery of novel enzymes and therapeutics. Directed evolution combines iterative rounds of mutagenesis and selection within living cells, offering a robust platform for engineering complex biological functions and improving protein stability. Future perspectives highlight the integration of machine learning and high-throughput sequencing technologies to enhance the efficiency and predictability of evolutionary trajectories in both in vitro and directed evolution approaches.
Ribosome display
Ribosome display, an in vitro evolution technique, enables rapid selection of high-affinity proteins from vast libraries without cellular transformation, offering advantages over traditional directed evolution methods reliant on in vivo screening.
Phage display
Phage display, a key technique in directed evolution, enables rapid in vitro evolution of proteins by linking phenotypes to genotypes on bacteriophage surfaces for efficient selection of high-affinity variants.
Error-prone PCR
Error-prone PCR in directed evolution introduces random mutations during DNA amplification to rapidly generate diverse enzyme variants, whereas in vitro evolution encompasses broader techniques including compartmentalized self-replication beyond error-prone PCR.
Compartmentalized self-replication
Compartmentalized self-replication in directed evolution enables high-throughput selection of enzyme variants by linking genotype and phenotype within microdroplets, offering greater efficiency and precision compared to traditional in vitro evolution methods.
DNA shuffling
DNA shuffling accelerates genetic diversity generation in directed evolution by recombining homologous sequences in vitro, enabling rapid optimization of biomolecules compared to traditional in vitro evolution methods.
SELEX (Systematic Evolution of Ligands by Exponential Enrichment)
SELEX, a key in vitro evolution technique, systematically selects high-affinity nucleic acid ligands through iterative binding, partitioning, and amplification, differing from broader directed evolution methods that typically involve random mutagenesis and screening of proteins or enzymes.
Mutagenesis libraries
Mutagenesis libraries in in vitro evolution offer higher diversity and precise control compared to directed evolution pools derived from iterative mutagenesis and selection cycles.
High-throughput screening
High-throughput screening in directed evolution accelerates the identification of improved enzyme variants by rapidly testing large mutant libraries, whereas in vitro evolution often relies on selection methods that may limit throughput and diversity exploration.
Fitness landscape mapping
In vitro evolution enables precise fitness landscape mapping by exploring vast genetic variants under controlled conditions, whereas directed evolution iteratively selects improved phenotypes in vivo or in vitro, offering practical fitness optimization but less detailed landscape resolution.
Genotype-phenotype linkage
In vitro evolution utilizes artificial genotype-phenotype linkage systems like ribosome display or mRNA display to screen vast molecular libraries, whereas directed evolution relies on iterative rounds of mutation and selection in living cells to maintain natural linkage between genotype and phenotype.
In vitro evolution vs Directed evolution Infographic
 njnir.com
njnir.com