Bioprinting enables the precise layer-by-layer construction of complex tissue structures using living cells, offering customizable 3D models for regenerative medicine and drug testing. Organ-on-a-chip technology replicates the microenvironment and physiological functions of human organs on a microfluidic platform, facilitating dynamic studies of organ-level responses and disease mechanisms. Both approaches complement each other by advancing personalized medicine through improved tissue modeling and functional analysis.
Table of Comparison
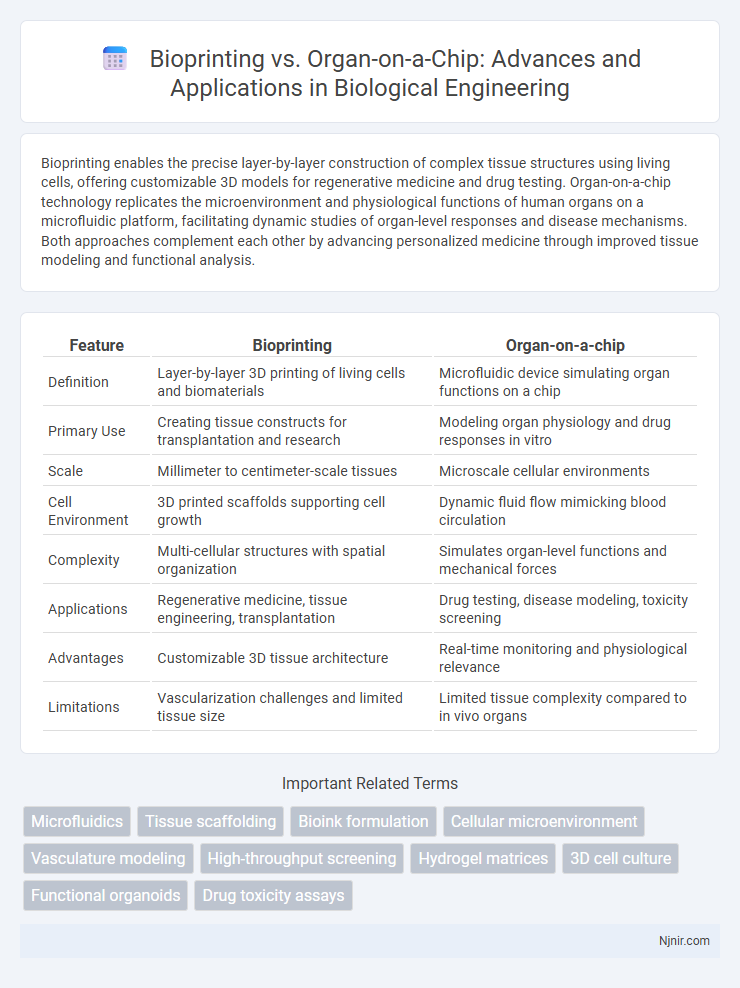
| Feature | Bioprinting | Organ-on-a-chip |
|---|---|---|
| Definition | Layer-by-layer 3D printing of living cells and biomaterials | Microfluidic device simulating organ functions on a chip |
| Primary Use | Creating tissue constructs for transplantation and research | Modeling organ physiology and drug responses in vitro |
| Scale | Millimeter to centimeter-scale tissues | Microscale cellular environments |
| Cell Environment | 3D printed scaffolds supporting cell growth | Dynamic fluid flow mimicking blood circulation |
| Complexity | Multi-cellular structures with spatial organization | Simulates organ-level functions and mechanical forces |
| Applications | Regenerative medicine, tissue engineering, transplantation | Drug testing, disease modeling, toxicity screening |
| Advantages | Customizable 3D tissue architecture | Real-time monitoring and physiological relevance |
| Limitations | Vascularization challenges and limited tissue size | Limited tissue complexity compared to in vivo organs |
Introduction to Bioprinting and Organ-on-a-Chip
Bioprinting uses layer-by-layer deposition of bioinks containing cells and biomaterials to create three-dimensional tissue structures that mimic natural organs. Organ-on-a-chip technology integrates living cells within microfluidic devices to simulate the physiological functions and microenvironments of human organs. Both techniques advance biomedical research by providing platforms for drug testing, disease modeling, and personalized medicine.
Evolution of Bioengineering Technologies
Bioprinting has revolutionized bioengineering by enabling precise, layer-by-layer fabrication of complex tissues using living cells and biomaterials, accelerating advancements in regenerative medicine and customized tissue models. Organ-on-a-chip technology, leveraging microfluidics and cell culture, mimics physiological microenvironments to simulate organ functions and disease mechanisms with high fidelity, enhancing drug discovery and toxicity testing. The evolution of these technologies reflects a shift towards more dynamic, scalable, and integrative platforms that combine 3D bioprinting's spatial accuracy with organ-on-a-chip's functional realism for improved biomedical applications.
Core Principles of Bioprinting
Bioprinting relies on precise layer-by-layer deposition of bioinks containing living cells and biomaterials to create 3D tissue constructs that mimic natural tissue architecture. This technique uses computer-aided design (CAD) models and various printing methods such as extrusion, inkjet, and laser-assisted printing to achieve spatial control over cell placement and matrix composition. Unlike organ-on-a-chip systems that simulate organ-level functions in microfluidic devices, bioprinting aims to fabricate scalable, structurally complex tissues with direct applications in regenerative medicine and tissue engineering.
Fundamental Concepts of Organ-on-a-Chip
Organ-on-a-chip technology integrates microfluidics and cell biology to recreate the functional units of human organs within a microscale device, enabling precise control over the cellular microenvironment and physiological responses. This system mimics organ-level functions, such as tissue-tissue interfaces and mechanical cues, facilitating high-throughput drug testing and disease modeling. Compared to bioprinting, organ-on-a-chip emphasizes dynamic biological processes over structural replication, offering real-time analysis of cellular interactions and tissue function.
Materials and Methods: A Comparative View
Bioprinting utilizes bioinks composed of hydrogels, living cells, and biomolecules, employing layer-by-layer additive manufacturing techniques to fabricate three-dimensional tissue constructs with precise spatial control. Organ-on-a-chip technology integrates microfluidic devices made from elastomeric polymers like PDMS, embedding living cells in microchannels to mimic tissue interfaces and dynamic biochemical environments. Both methods rely on advanced materials science and cell culture protocols, but bioprinting emphasizes structural complexity, while organ-on-a-chip prioritizes physiological functionality and real-time cellular responses.
Applications in Drug Discovery and Testing
Bioprinting enables the creation of 3D tissue models with precise cellular architecture, facilitating high-throughput drug screening and personalized medicine development. Organ-on-a-chip technology mimics organ-level functions using microfluidic channels and living cells, providing dynamic environments for real-time drug toxicity and efficacy testing. Both technologies enhance predictive accuracy in pharmacokinetics and pharmacodynamics, reducing reliance on animal models and accelerating drug discovery pipelines.
Advantages and Limitations of Bioprinting
Bioprinting offers precise spatial control for fabricating complex tissue structures, enabling the creation of patient-specific models and potential organ replacements with high structural fidelity. Its main limitation lies in replicating the full vascularization and physiological functions of native tissues, often resulting in challenges with long-term viability and integration. While bioprinting excels in customization and scalability, it requires further advancements to overcome issues related to material compatibility and functional maturation.
Strengths and Constraints of Organ-on-a-Chip
Organ-on-a-chip technology offers high-precision simulation of human organ functions using microfluidic channels that replicate the cellular environment, enabling dynamic study of drug responses and disease models with minimal reagent use. Its strengths include real-time monitoring, scalability for high-throughput screening, and reduced reliance on animal testing, making it ideal for personalized medicine and toxicity assessment. Constraints involve limited long-term viability of cultured cells, challenges in replicating full organ complexity, and technical difficulties in integrating multiple organ systems for systemic studies.
Future Trends and Integration Opportunities
Bioprinting advances toward creating fully functional, vascularized tissues with enhanced biocompatibility, driving more realistic organ models for drug testing and transplantation. Organ-on-a-chip technology is evolving through improved microfluidic precision and real-time biosensing, enabling dynamic simulation of physiological environments. Integrating bioprinting with organ-on-a-chip platforms offers the potential to combine 3D structural complexity with controlled microenvironments, revolutionizing personalized medicine and accelerating drug discovery pipelines.
Conclusion: Impact on Personalized Medicine
Bioprinting enables the fabrication of patient-specific tissues with precise cellular architecture, advancing personalized medicine through customized implants and drug screening. Organ-on-a-chip technology provides dynamic, microfluidic models that replicate individual organ responses, enhancing disease modeling and tailored therapeutics. Integrating both technologies accelerates the development of personalized treatments by combining structural complexity with functional accuracy in patient-specific scenarios.
Microfluidics
Microfluidics enhances organ-on-a-chip technology by precisely controlling fluid flow to mimic physiological conditions, whereas bioprinting uses layer-by-layer deposition of bioinks to create complex 3D tissue structures.
Tissue scaffolding
Bioprinting uses 3D-printed tissue scaffolds to create complex, customizable tissue structures, while organ-on-a-chip relies on microfluidic platforms with biomimetic scaffolds to simulate organ-level functions.
Bioink formulation
Bioink formulation in bioprinting involves customizing biomaterials for cell viability and structural integrity, whereas organ-on-a-chip biofabrication prioritizes microfluidic compatibility and precise extracellular matrix mimicry.
Cellular microenvironment
Bioprinting precisely constructs 3D cellular microenvironments by layering biomaterials and cells, while organ-on-a-chip replicates dynamic microfluidic conditions to mimic physiological cellular interactions.
Vasculature modeling
Bioprinting enables precise, three-dimensional vasculature modeling for organ tissue engineering, while organ-on-a-chip technology offers microfluidic platforms that simulate dynamic vascular flow and physiological responses.
High-throughput screening
Bioprinting enables high-throughput screening by fabricating customizable 3D tissue models, while organ-on-a-chip offers dynamic microenvironment control for scalable drug testing.
Hydrogel matrices
Hydrogel matrices in bioprinting provide customizable 3D scaffolds for cell growth, whereas in organ-on-a-chip systems, they enable microenvironment simulation and dynamic tissue interactions for precise physiological modeling.
3D cell culture
Bioprinting enables precise 3D cell culture construction for tissue fabrication, while organ-on-a-chip platforms replicate dynamic microenvironments for enhanced cell functionality and disease modeling.
Functional organoids
Bioprinting enables precise spatial arrangement of multiple cell types to create complex, functional organoids, while organ-on-a-chip technology replicates organ-level physiological functions through microfluidic systems for dynamic testing and modeling.
Drug toxicity assays
Bioprinting creates three-dimensional tissue models with precise cellular architecture for enhanced drug toxicity assays, while organ-on-a-chip systems simulate dynamic physiological conditions to improve predictive accuracy in drug safety testing.
Bioprinting vs Organ-on-a-chip Infographic
 njnir.com
njnir.com