Aptamer selection offers a rapid and cost-effective alternative to antibody generation by utilizing in vitro techniques that enable precise targeting of specific molecules. Unlike antibodies, aptamers are synthetic nucleic acid ligands with high stability, low immunogenicity, and ease of modification, enhancing their applicability in diverse biological engineering applications. This adaptability allows aptamers to outperform antibodies in therapeutic, diagnostic, and biosensing platforms while reducing ethical concerns linked to animal use.
Table of Comparison
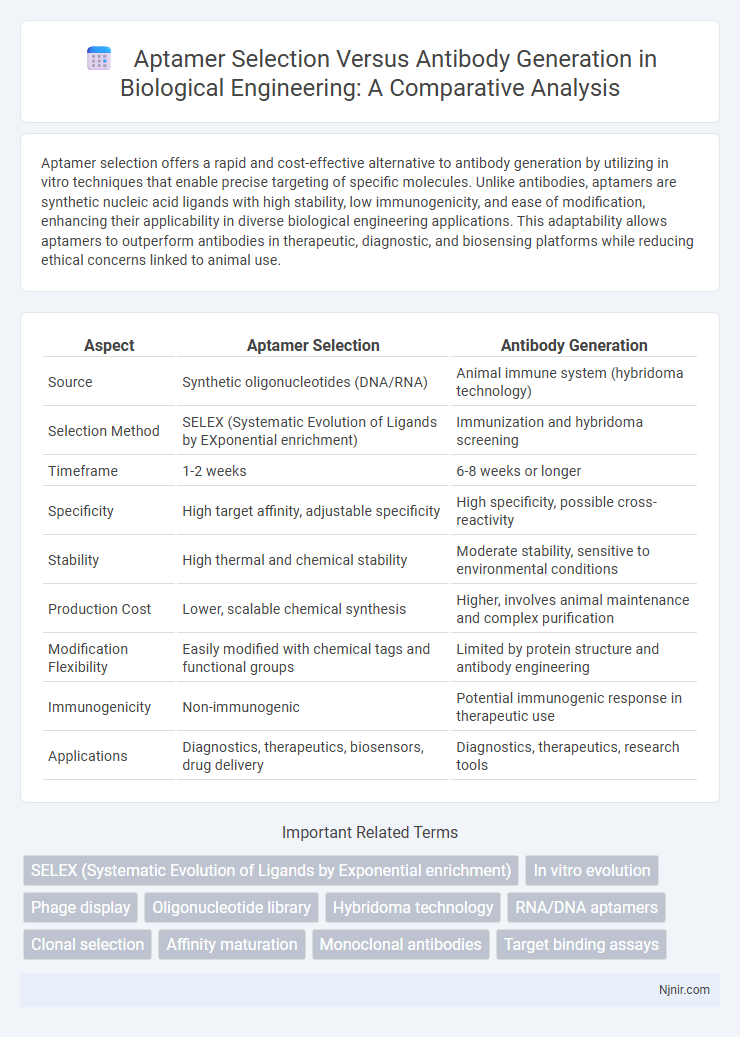
| Aspect | Aptamer Selection | Antibody Generation |
|---|---|---|
| Source | Synthetic oligonucleotides (DNA/RNA) | Animal immune system (hybridoma technology) |
| Selection Method | SELEX (Systematic Evolution of Ligands by EXponential enrichment) | Immunization and hybridoma screening |
| Timeframe | 1-2 weeks | 6-8 weeks or longer |
| Specificity | High target affinity, adjustable specificity | High specificity, possible cross-reactivity |
| Stability | High thermal and chemical stability | Moderate stability, sensitive to environmental conditions |
| Production Cost | Lower, scalable chemical synthesis | Higher, involves animal maintenance and complex purification |
| Modification Flexibility | Easily modified with chemical tags and functional groups | Limited by protein structure and antibody engineering |
| Immunogenicity | Non-immunogenic | Potential immunogenic response in therapeutic use |
| Applications | Diagnostics, therapeutics, biosensors, drug delivery | Diagnostics, therapeutics, research tools |
Introduction to Molecular Recognition Elements
Molecular recognition elements like aptamers and antibodies are crucial for specific target binding in diagnostics and therapeutics. Aptamer selection involves in vitro processes such as SELEX (Systematic Evolution of Ligands by Exponential Enrichment) to isolate nucleic acid sequences with high affinity and specificity for a target molecule. Antibody generation typically relies on in vivo immunization to produce proteins that recognize antigens, offering high specificity but with longer production times and batch variability.
Overview of Aptamer Selection Techniques
Aptamer selection techniques primarily involve Systematic Evolution of Ligands by EXponential enrichment (SELEX), which iteratively enriches high-affinity nucleic acid ligands against target molecules through binding, separation, and amplification cycles. Variations such as Cell-SELEX enable targeting whole cells, while Magnetic-bead SELEX enhances efficiency by immobilizing targets for easier separation. Compared to antibody generation, aptamer selection offers rapid synthesis, high specificity, chemical stability, and ease of modification without the need for animal hosts.
Fundamentals of Antibody Generation
Antibody generation involves the immunization of animals with specific antigens to stimulate B-cell activation and clonal expansion, producing high-affinity antibodies. The process includes hybridoma technology or phage display libraries for monoclonal antibody production, ensuring specificity and binding strength. Key fundamentals include antigen design, immune response modulation, and rigorous screening to isolate target-specific antibodies for therapeutic or diagnostic applications.
Comparative Timeline: Aptamer vs Antibody Development
Aptamer selection typically requires 1 to 4 weeks through systematic evolution of ligands by exponential enrichment (SELEX), whereas antibody generation via hybridoma technology or recombinant methods takes 3 to 6 months. Aptamers benefit from in vitro synthesis and rapid optimization cycles, enabling faster development compared to the in vivo immunization processes needed for antibodies. The shorter timeline for aptamer development accelerates applications in diagnostics and therapeutics where rapid target-specific binding agents are essential.
Specificity and Affinity: Evaluating Binding Efficiency
Aptamer selection employs SELEX technology to achieve high specificity and affinity by iterative binding and amplification steps, enabling precise target recognition at the molecular level. Antibody generation relies on immune response mechanisms to produce proteins with variable affinity and specificity, often influenced by antigen complexity and host variability. Comparative studies reveal aptamers typically exhibit lower batch-to-batch variation and can reach nanomolar to picomolar binding affinities, while antibodies may provide stronger affinity but with greater production inconsistency.
Stability and Storage Considerations
Aptamers exhibit superior stability compared to antibodies, maintaining functionality under a wide range of temperatures and pH conditions, which reduces the need for stringent cold chain storage. Antibodies, being protein-based, often require refrigeration or freezing to preserve their structural integrity and binding affinity over time. This enhanced thermal stability and easier storage make aptamers particularly advantageous for applications in resource-limited settings or long-term storage scenarios.
Cost-Effectiveness in Production
Aptamer selection offers a more cost-effective production process compared to antibody generation due to its in vitro synthesis, eliminating the need for animals and reducing batch-to-batch variability. The chemical synthesis of aptamers enables rapid, scalable manufacturing with lower resource consumption and shorter development timelines. In contrast, antibody generation involves complex cell culture or animal immunization, resulting in higher costs and longer production cycles.
Applications in Diagnostics and Therapeutics
Aptamer selection offers rapid, cost-effective synthesis with high specificity, making it ideal for diagnostic assays such as biosensors and point-of-care testing. Antibody generation, while more time-consuming and expensive, provides robust affinity and versatility for therapeutic applications including monoclonal antibody drugs and immunotherapies. Both aptamers and antibodies are pivotal in precision medicine, with aptamers excelling in stability and modifications, and antibodies leading in clinical success and regulatory approval.
Scalability and Engineering Flexibility
Aptamer selection offers superior scalability through rapid, automated in vitro processes that enable high-throughput synthesis and modification, contrasting with the time-consuming and resource-intensive animal immunization required for antibody generation. Engineering flexibility is enhanced in aptamers by precise chemical synthesis allowing easy incorporation of functional groups, structural modifications, and conjugations, whereas antibodies depend on complex protein engineering techniques with limited modification capacity. The scalability and customizable nature of aptamers make them ideal for applications demanding large-scale production and tailored molecular recognition properties.
Future Prospects in Biological Engineering
Aptamer selection offers faster development cycles and higher batch-to-batch consistency compared to traditional antibody generation, making it a promising tool for future biological engineering applications. The chemical synthesis of aptamers enables easy modification and increased stability, supporting advanced therapeutic and diagnostic technologies. Integrating aptamer-based systems with synthetic biology and nanotechnology could revolutionize targeted drug delivery and biosensing platforms.
SELEX (Systematic Evolution of Ligands by Exponential enrichment)
SELEX enables precise aptamer selection through iterative binding, partitioning, and amplification cycles to isolate high-affinity nucleic acid ligands, offering a faster, more cost-effective, and versatile alternative to conventional antibody generation.
In vitro evolution
In vitro evolution enables aptamer selection through iterative rounds of binding, separation, and amplification, offering faster, more specific, and chemically modifiable alternatives to traditional antibody generation.
Phage display
Phage display enables rapid, high-throughput selection of antibodies by expressing diverse peptide libraries on bacteriophage surfaces, whereas aptamer selection typically involves iterative in vitro SELEX processes to isolate nucleic acid ligands with high specificity.
Oligonucleotide library
Oligonucleotide libraries in aptamer selection offer higher diversity and rapid synthesis compared to antibody generation, enabling more efficient targeting of specific molecules.
Hybridoma technology
Hybridoma technology enables antibody generation by fusing B cells with myeloma cells to produce monoclonal antibodies, whereas aptamer selection uses in vitro SELEX processes to identify nucleic acid ligands with high target specificity.
RNA/DNA aptamers
RNA/DNA aptamer selection offers faster, more cost-effective, and highly specific targeting compared to traditional antibody generation, enabling versatile applications in diagnostics and therapeutics.
Clonal selection
Clonal selection during aptamer selection involves iterative rounds of binding, amplification, and enrichment to isolate high-affinity nucleic acid ligands, whereas antibody generation relies on clonal expansion of B cells producing specific immunoglobulins.
Affinity maturation
Aptamer selection enables rapid in vitro affinity maturation through iterative SELEX cycles, while antibody generation relies on in vivo immune responses and subsequent hybridoma screening for improved affinity.
Monoclonal antibodies
Monoclonal antibody generation involves immunizing animals to produce highly specific antibodies through hybridoma technology, whereas aptamer selection relies on in vitro SELEX processes to develop synthetic nucleic acid ligands with comparable specificity and affinity but enhanced stability and reduced immunogenicity.
Target binding assays
Target binding assays for aptamer selection demonstrate higher specificity and reproducibility compared to antibody generation methods, enhancing diagnostic and therapeutic accuracy.
Aptamer selection vs Antibody generation Infographic
 njnir.com
njnir.com