High-throughput screening enables rapid testing of thousands of biological samples, accelerating the identification of key genetic or chemical compounds with desired properties. Single-cell analysis provides detailed insights into cellular heterogeneity by examining individual cells at the molecular level, revealing variations that bulk screening might miss. Combining both techniques enhances biological engineering outcomes by maximizing data throughput while capturing critical single-cell variations.
Table of Comparison
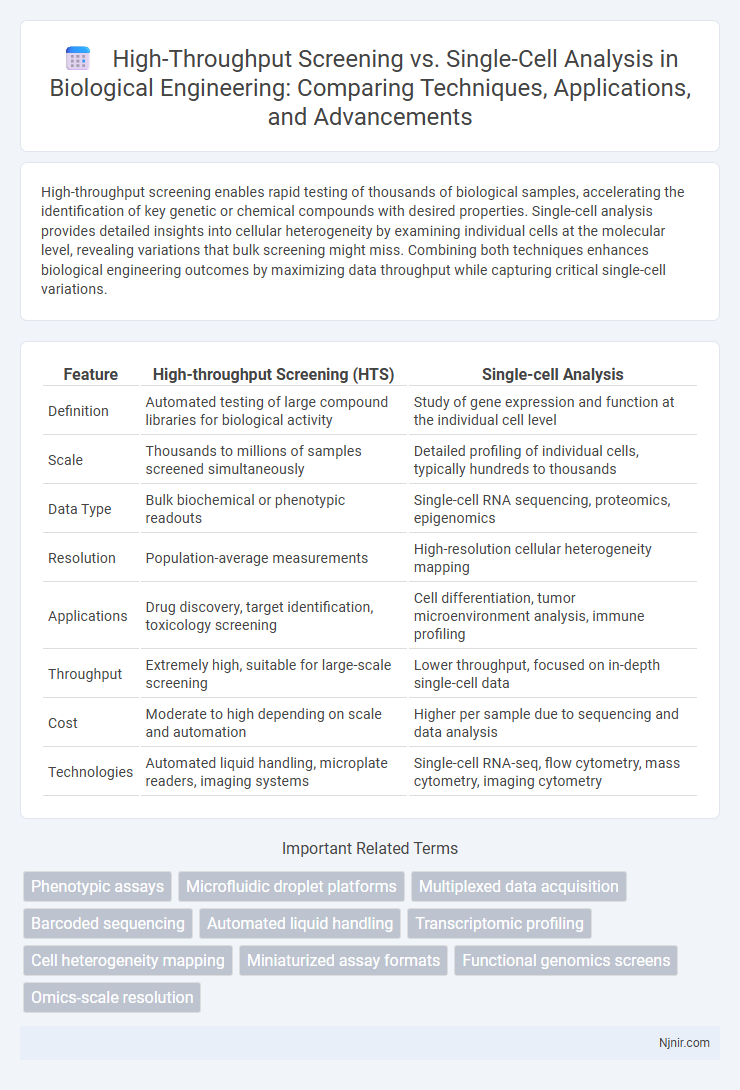
| Feature | High-throughput Screening (HTS) | Single-cell Analysis |
|---|---|---|
| Definition | Automated testing of large compound libraries for biological activity | Study of gene expression and function at the individual cell level |
| Scale | Thousands to millions of samples screened simultaneously | Detailed profiling of individual cells, typically hundreds to thousands |
| Data Type | Bulk biochemical or phenotypic readouts | Single-cell RNA sequencing, proteomics, epigenomics |
| Resolution | Population-average measurements | High-resolution cellular heterogeneity mapping |
| Applications | Drug discovery, target identification, toxicology screening | Cell differentiation, tumor microenvironment analysis, immune profiling |
| Throughput | Extremely high, suitable for large-scale screening | Lower throughput, focused on in-depth single-cell data |
| Cost | Moderate to high depending on scale and automation | Higher per sample due to sequencing and data analysis |
| Technologies | Automated liquid handling, microplate readers, imaging systems | Single-cell RNA-seq, flow cytometry, mass cytometry, imaging cytometry |
Overview of High-Throughput Screening in Biological Engineering
High-throughput screening (HTS) in biological engineering enables rapid testing of thousands to millions of biological samples using automated platforms, significantly accelerating drug discovery and functional genomics research. This approach leverages robotics, data processing algorithms, and sensitive detectors to identify candidate molecules or genetic modifications affecting biological pathways. HTS contrasts with single-cell analysis by providing population-level data, emphasizing scalability and speed for initial compound or gene target identification.
Principles and Techniques in Single-Cell Analysis
Single-cell analysis employs techniques like flow cytometry, single-cell RNA sequencing (scRNA-seq), and mass cytometry to measure gene expression and protein levels at the individual cell level, revealing cellular heterogeneity. High-throughput screening (HTS) relies on automated robotic systems and multi-well plates to quickly test thousands of chemical or genetic perturbations but lacks single-cell resolution. The core principle of single-cell analysis is isolating and profiling individual cells to understand complex biological systems, enabling precise identification of cell states and rare subpopulations missed by bulk HTS methods.
Comparative Sensitivity: Bulk Versus Single-Cell Approaches
High-throughput screening offers robust sensitivity in detecting bulk population-level responses, enabling rapid identification of biochemical or genetic modulators across thousands of samples. Single-cell analysis, by contrast, provides exceptional sensitivity at the individual cell level, uncovering cellular heterogeneity and rare subpopulations undetectable in bulk assays. This comparative sensitivity difference highlights single-cell techniques as essential for precise biomarker discovery and understanding complex tissue dynamics.
Throughput Capacity: Scalability and Efficiency
High-throughput screening (HTS) enables rapid assessment of thousands to millions of compounds or genetic elements with exceptional scalability, making it highly efficient for large-scale drug discovery and functional genomics. Single-cell analysis focuses on resolving cellular heterogeneity at an individual cell level, providing detailed insights but generally operates with lower throughput compared to HTS due to the complexity of isolating and processing single cells. Advances in microfluidics and automation are bridging the throughput gap, enhancing the scalability and efficiency of single-cell techniques while maintaining high-resolution data.
Data Complexity and Interpretation Challenges
High-throughput screening generates vast, bulk data sets enabling rapid identification of potential targets but often masks cellular heterogeneity, complicating data interpretation. Single-cell analysis captures gene expression and signaling at an individual cell level, revealing heterogeneity but producing high-dimensional data that require advanced computational methods and statistical models for meaningful interpretation. The complexity in single-cell data demands integration of multi-omics and robust bioinformatics pipelines to decode cellular states, whereas high-throughput screening focuses on scalable, reproducible output with less granularity.
Applications in Synthetic Biology and Genetic Engineering
High-throughput screening accelerates the identification of functional genetic variants and engineered enzymes by rapidly testing thousands of biological samples, thus enabling scalable synthetic biology applications such as pathway optimization and strain improvement. Single-cell analysis provides detailed insights into cellular heterogeneity and gene expression dynamics, crucial for precise genetic engineering and fine-tuning synthetic circuits at an individual cell level. Combining these techniques enhances synthetic biology workflows by coupling large-scale screening efficiency with single-cell resolution for optimized genetic modifications.
Integration of Automation and Microfluidics
High-throughput screening employs advanced automation and microfluidics to process thousands of samples rapidly, enhancing drug discovery and biomarker identification efficiency. Single-cell analysis integrates microfluidic platforms with automated systems to isolate and analyze individual cells, providing precise cellular heterogeneity insights. The combination of automation and microfluidics in both techniques accelerates data acquisition while minimizing sample volume and human error.
Cost-Efficiency and Resource Considerations
High-throughput screening enables rapid testing of thousands of compounds with lower per-sample cost, making it highly cost-efficient for drug discovery and large-scale assays. Single-cell analysis, while providing detailed cellular heterogeneity insights, requires more expensive reagents, specialized equipment, and longer processing times, increasing overall resource investment. Balancing cost-efficiency, high-throughput screening suits broad initial screens, whereas single-cell analysis is ideal for in-depth characterization despite higher expenses.
Advancements in Multi-Omics Profiling
High-throughput screening accelerates drug discovery by rapidly testing large compound libraries, while single-cell analysis offers detailed insights into cellular heterogeneity. Recent advancements in multi-omics profiling integrate genomics, transcriptomics, proteomics, and metabolomics at the single-cell level, enabling comprehensive characterization of individual cell states within complex tissues. This fusion enhances precision medicine by uncovering unique biomarkers and therapeutic targets that high-throughput methods alone may overlook.
Future Directions and Emerging Trends
High-throughput screening continues to evolve with the integration of artificial intelligence and machine learning algorithms to accelerate drug discovery and biomarker identification. Single-cell analysis is advancing through multi-omics approaches and spatial transcriptomics, enabling comprehensive cellular heterogeneity mapping in complex tissues. Emerging trends include combining high-throughput and single-cell technologies to enhance precision medicine and uncover novel therapeutic targets.
Phenotypic assays
High-throughput screening enables large-scale phenotypic assays by rapidly testing thousands of compounds for biological activity, while single-cell analysis provides detailed phenotypic insights at the individual cell level, enhancing understanding of cellular heterogeneity.
Microfluidic droplet platforms
Microfluidic droplet platforms enable high-throughput screening by encapsulating thousands of single cells in uniform droplets, facilitating precise single-cell analysis with increased sensitivity and throughput compared to bulk methods.
Multiplexed data acquisition
High-throughput screening enables rapid multiplexed data acquisition across thousands of samples, while single-cell analysis provides detailed multiplexed molecular profiles at the individual cell level for precise cellular heterogeneity characterization.
Barcoded sequencing
Barcoded sequencing enables high-throughput screening by simultaneously analyzing thousands of individual cells, surpassing traditional single-cell analysis in scalability and precision.
Automated liquid handling
Automated liquid handling enhances high-throughput screening by rapidly processing thousands of samples for drug discovery, while in single-cell analysis, it precisely manipulates individual cells to enable detailed molecular profiling and reduce sample variability.
Transcriptomic profiling
High-throughput screening enables rapid transcriptomic profiling of numerous samples for broad gene expression patterns, whereas single-cell analysis provides detailed transcriptomic profiles at the individual cell level, revealing cellular heterogeneity and rare cell populations.
Cell heterogeneity mapping
High-throughput screening enables rapid identification of cell populations while single-cell analysis provides detailed mapping of cell heterogeneity at the individual cell level.
Miniaturized assay formats
Miniaturized assay formats in high-throughput screening enable rapid, cost-effective testing of thousands of compounds, whereas single-cell analysis miniaturization allows detailed profiling of individual cell heterogeneity with enhanced spatial resolution.
Functional genomics screens
High-throughput screening enables large-scale functional genomics screens by rapidly testing thousands of genetic perturbations, while single-cell analysis provides detailed insights into cellular heterogeneity and gene function at an individual cell level.
Omics-scale resolution
High-throughput screening offers broad Omics-scale resolution for large sample sets, while single-cell analysis provides detailed Omics resolution at the individual cell level, enabling precise cellular heterogeneity profiling.
High-throughput screening vs Single-cell analysis Infographic
 njnir.com
njnir.com