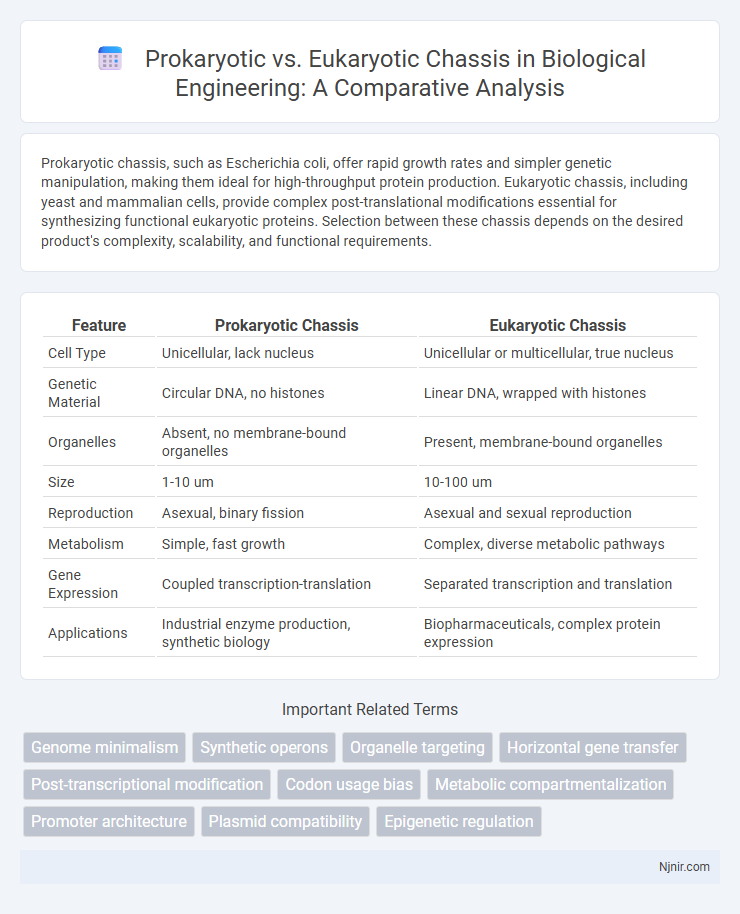
Prokaryotic chassis, such as Escherichia coli, offer rapid growth rates and simpler genetic manipulation, making them ideal for high-throughput protein production. Eukaryotic chassis, including yeast and mammalian cells, provide complex post-translational modifications essential for synthesizing functional eukaryotic proteins. Selection between these chassis depends on the desired product's complexity, scalability, and functional requirements.
Table of Comparison
| Feature | Prokaryotic Chassis | Eukaryotic Chassis |
|---|---|---|
| Cell Type | Unicellular, lack nucleus | Unicellular or multicellular, true nucleus |
| Genetic Material | Circular DNA, no histones | Linear DNA, wrapped with histones |
| Organelles | Absent, no membrane-bound organelles | Present, membrane-bound organelles |
| Size | 1-10 um | 10-100 um |
| Reproduction | Asexual, binary fission | Asexual and sexual reproduction |
| Metabolism | Simple, fast growth | Complex, diverse metabolic pathways |
| Gene Expression | Coupled transcription-translation | Separated transcription and translation |
| Applications | Industrial enzyme production, synthetic biology | Biopharmaceuticals, complex protein expression |
Introduction to Biological Chassis Systems
Prokaryotic chassis, such as Escherichia coli, offer simplicity with a single circular chromosome and lack of organelles, enabling efficient genetic manipulation for synthetic biology applications. In contrast, eukaryotic chassis like Saccharomyces cerevisiae possess complex cellular organization, including a nucleus and membrane-bound organelles, facilitating advanced cellular processes and post-translational modifications. Selection between prokaryotic and eukaryotic chassis depends on experimental goals, with prokaryotes favored for rapid growth and ease of use, while eukaryotes support more intricate biological functions.
Defining Prokaryotic and Eukaryotic Chassis
Prokaryotic chassis are cellular platforms derived from prokaryotes, primarily bacteria, characterized by lacking a membrane-bound nucleus and organelles, allowing simpler and faster genetic manipulation. Eukaryotic chassis originate from eukaryotic cells, such as yeast or mammalian cells, featuring a complex cellular architecture with a defined nucleus and diverse organelles that enable sophisticated metabolic processes and protein expression. The fundamental differences in cellular complexity and genetic organization between prokaryotic and eukaryotic chassis influence their suitability for various synthetic biology applications.
Genetic Architecture and Complexity
Prokaryotic chassis feature simpler genetic architecture with a single circular chromosome located in the nucleoid region, lacking membrane-bound organelles, which facilitates rapid gene expression and metabolic efficiency. Eukaryotic chassis possess complex genetic organization with multiple linear chromosomes housed within a membrane-bound nucleus, accompanied by extensive regulatory elements, introns, and compartmentalized cellular structures. This complexity enables advanced gene regulation, spatiotemporal expression control, and functional specialization essential for multicellular development and diverse cellular processes.
Metabolic Flexibility and Pathway Integration
Prokaryotic chassis exhibit high metabolic flexibility due to their simpler cellular organization and diverse metabolic pathways, enabling efficient adaptation to varying environmental conditions. In contrast, eukaryotic chassis possess compartmentalized organelles that allow complex pathway integration and regulation but may reduce overall metabolic adaptability. Engineering prokaryotic systems often targets rapid pathway modifications for bioproduction, while eukaryotic chassis support intricate metabolic engineering for multi-step biosynthesis and product complexity.
Scalability and Growth Rates
Prokaryotic chassis, such as Escherichia coli, exhibit rapid growth rates and high scalability due to their simple cellular structure and efficient metabolic pathways, enabling quick replication and easy genetic manipulation. Eukaryotic chassis, including Saccharomyces cerevisiae, grow slower but offer more complex post-translational modifications and compartmentalization, which can limit scalability but enhance functional diversity. The choice between prokaryotic and eukaryotic chassis depends on balancing rapid biomass accumulation with sophisticated protein expression needs in biotechnological applications.
Engineering Tools and Techniques
Prokaryotic chassis, primarily represented by bacteria like Escherichia coli, benefit from well-established genetic engineering tools such as CRISPR-Cas systems, plasmid vectors, and lambda red recombination, enabling precise genome editing and rapid cloning. Eukaryotic chassis, including yeast and mammalian cells, utilize advanced techniques like homologous recombination, RNA interference, and synthetic biology platforms that accommodate complex post-translational modifications and compartmentalized cellular processes. The choice of chassis dictates the engineering toolbox, where prokaryotes offer simplicity and speed for genetic manipulation, while eukaryotic systems provide sophisticated regulatory control and functional diversity.
Tolerance to Environmental Stresses
Prokaryotic chassis such as Escherichia coli exhibit high tolerance to extreme environmental stresses including pH fluctuations, temperature variations, and osmotic pressure due to their robust cell wall and simpler genome enabling rapid adaptation. In contrast, eukaryotic chassis like Saccharomyces cerevisiae possess compartmentalized organelles and intricate stress response pathways, conferring enhanced resilience to oxidative stress and nutrient deprivation. The choice between prokaryotic and eukaryotic chassis depends on the specific environmental stress factors encountered in industrial bioprocessing or synthetic biology applications.
Applications in Synthetic Biology
Prokaryotic chassis, such as Escherichia coli, are widely used in synthetic biology for rapid gene expression, metabolic engineering, and production of biomolecules due to their simple genetic architecture and fast growth rates. Eukaryotic chassis, including Saccharomyces cerevisiae and mammalian cells, enable more complex protein folding, post-translational modifications, and functional expression of eukaryotic pathways essential for pharmaceuticals and therapeutic proteins. The choice between prokaryotic and eukaryotic chassis depends on the specific synthetic biology application requirements, including complexity of biological functions and scalability.
Limitations and Biosecurity Considerations
Prokaryotic chassis, such as Escherichia coli, face limitations including reduced post-translational modification capabilities and simpler metabolic pathways, restricting their use in complex protein expression. Eukaryotic chassis like Saccharomyces cerevisiae offer advanced cellular machinery but present challenges in genome complexity and slower growth rates, impacting scalability. Biosecurity considerations for prokaryotic systems emphasize horizontal gene transfer risks and containment strategies, while eukaryotic systems require evaluation of potential pathogenicity and environmental persistence.
Future Perspectives in Chassis Development
Advancements in synthetic biology are driving the development of prokaryotic chassis such as Escherichia coli for rapid and cost-effective bioproduction, benefiting from their simple genome and fast growth rates. Eukaryotic chassis like Saccharomyces cerevisiae offer complex post-translational modifications and compartmentalization, essential for producing pharmaceuticals and biofuels with higher fidelity. Future perspectives emphasize integrating genome editing tools like CRISPR-Cas9 and machine learning to enhance chassis precision, scalability, and functional diversity across both prokaryotic and eukaryotic platforms.
Genome minimalism
Prokaryotic chassis exhibit genome minimalism with compact, streamlined DNA lacking introns and extensive non-coding regions, contrasting with the larger, more complex genomes of eukaryotic chassis containing numerous introns and regulatory elements.
Synthetic operons
Synthetic operons in prokaryotic chassis enable efficient multi-gene expression within compact genomes, whereas eukaryotic chassis require complex regulatory elements for coordinated expression due to their compartmentalized cellular structures.
Organelle targeting
Prokaryotic chassis lack membrane-bound organelles, limiting precise organelle targeting compared to eukaryotic chassis, which contain specialized organelles enabling efficient compartmentalization and targeted gene expression.
Horizontal gene transfer
Horizontal gene transfer occurs more frequently and efficiently in prokaryotic chassis than in eukaryotic chassis due to simpler cell structures and mechanisms like conjugation, transformation, and transduction.
Post-transcriptional modification
Prokaryotic chassis lack extensive post-transcriptional modifications compared to eukaryotic chassis, which exhibit complex processes like splicing, 5' capping, and polyadenylation essential for mRNA maturation and regulation.
Codon usage bias
Prokaryotic chassis exhibit strong codon usage bias optimizing translation efficiency for rapid growth, while eukaryotic chassis display more complex and variable codon preferences influenced by diverse cellular processes and tissue-specific expression.
Metabolic compartmentalization
Prokaryotic chassis exhibit limited metabolic compartmentalization due to the absence of membrane-bound organelles, whereas eukaryotic chassis possess extensive metabolic compartmentalization within organelles like mitochondria and chloroplasts, enhancing metabolic efficiency and regulation.
Promoter architecture
Prokaryotic chassis feature simpler promoter architectures with conserved -10 and -35 consensus sequences facilitating direct RNA polymerase binding, whereas eukaryotic chassis possess complex promoters containing core elements like TATA boxes, enhancers, and multiple regulatory motifs guiding sophisticated transcription initiation by RNA polymerase II.
Plasmid compatibility
Prokaryotic chassis typically exhibit higher plasmid compatibility due to simpler replication origins and fewer post-transcriptional modifications compared to eukaryotic chassis, which often require specialized vectors for efficient plasmid maintenance.
Epigenetic regulation
Prokaryotic chassis lack complex epigenetic regulation mechanisms such as histone modification and DNA methylation patterns found in eukaryotic chassis, limiting their ability to modulate gene expression dynamically.
Prokaryotic chassis vs Eukaryotic chassis Infographic
 njnir.com
njnir.com