Biofoundries revolutionize biological engineering by automating the design-build-test-learn cycle, significantly accelerating synthetic biology workflows compared to traditional labs. They integrate robotics, advanced software, and high-throughput screening to enhance precision and reproducibility in genetic manipulation and metabolic engineering. Traditional labs often rely on manual techniques that are time-consuming and prone to variability, limiting scalability and efficiency in complex bioengineering projects.
Table of Comparison
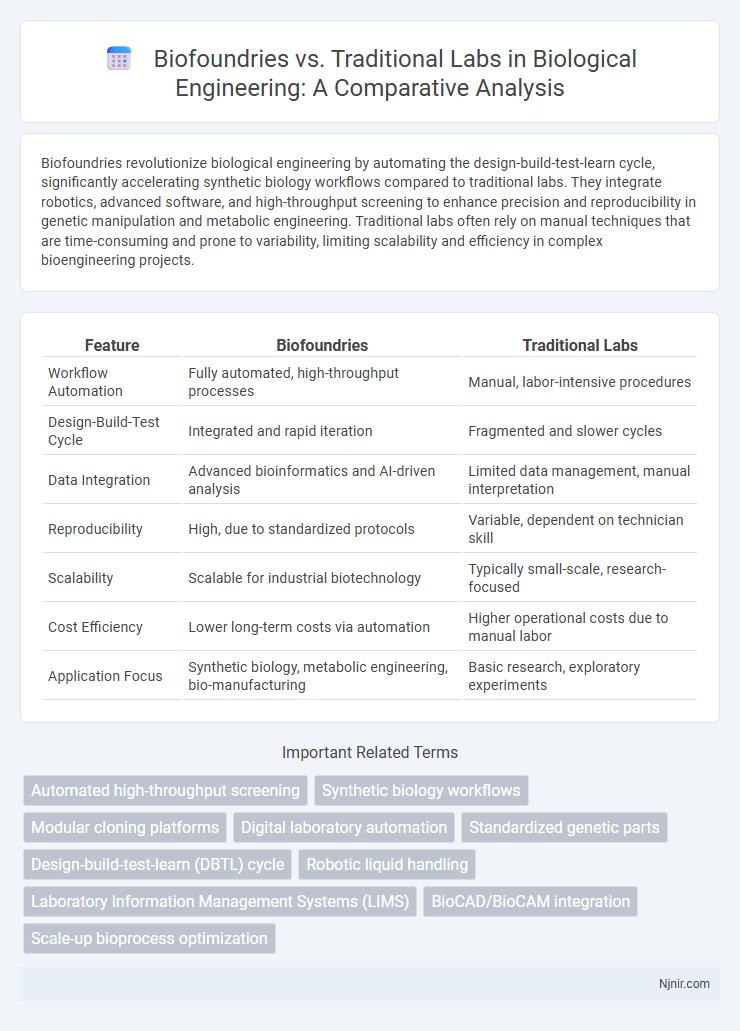
| Feature | Biofoundries | Traditional Labs |
|---|---|---|
| Workflow Automation | Fully automated, high-throughput processes | Manual, labor-intensive procedures |
| Design-Build-Test Cycle | Integrated and rapid iteration | Fragmented and slower cycles |
| Data Integration | Advanced bioinformatics and AI-driven analysis | Limited data management, manual interpretation |
| Reproducibility | High, due to standardized protocols | Variable, dependent on technician skill |
| Scalability | Scalable for industrial biotechnology | Typically small-scale, research-focused |
| Cost Efficiency | Lower long-term costs via automation | Higher operational costs due to manual labor |
| Application Focus | Synthetic biology, metabolic engineering, bio-manufacturing | Basic research, exploratory experiments |
Introduction: Defining Biofoundries and Traditional Labs
Biofoundries are automated, high-throughput facilities designed for synthetic biology, integrating robotics, software, and standardized biological parts to accelerate genetic engineering. Traditional labs rely on manual techniques, skilled technicians, and conventional equipment for experimentation and research in biology. The contrast centers on scalability, efficiency, and reproducibility, with biofoundries delivering faster design-build-test cycles compared to the labor-intensive processes in traditional labs.
Core Technologies: Automation and Digitalization in Biofoundries
Biofoundries leverage advanced automation technologies including robotic liquid handlers, high-throughput sequencing, and microfluidic devices, enabling rapid, precise, and repeatable biological experimentation far beyond traditional manual methods. Digitalization in biofoundries integrates laboratory information management systems (LIMS), computational design tools, and machine learning algorithms to optimize experimental workflows and data analysis, enhancing reproducibility and accelerating bioengineering cycles. This convergence of automation and digital technologies transforms biofoundries into scalable, efficient hubs for synthetic biology, contrasting with the lower throughput and higher variability seen in conventional laboratories.
Traditional Lab Workflows: Manual Processes and Flexibility
Traditional lab workflows rely heavily on manual processes such as pipetting, sample preparation, and data recording, which can increase the risk of human error and limit throughput. These manual methods offer high flexibility, allowing scientists to easily adapt protocols and experiment designs based on real-time observations and specific research needs. However, this flexibility often comes at the cost of scalability and reproducibility compared to the automated systems found in biofoundries.
Throughput and Scalability: High-Volume vs. Small-Scale Experiments
Biofoundries enable high-throughput processing by automating design-build-test-learn cycles, allowing thousands of genetic constructs to be tested simultaneously. Traditional labs typically rely on manual techniques, limiting scalability and throughput to small-scale, time-intensive experiments. This fundamental difference positions biofoundries as essential platforms for large-scale synthetic biology projects requiring rapid iteration and optimization.
Customization and Standardization: Protocols Across Platforms
Biofoundries excel in customization by enabling automated, high-throughput workflows tailored to specific genetic constructs, whereas traditional labs rely on manual, labor-intensive protocols that limit scalability. Standardization in biofoundries ensures reproducibility across platforms through integrated software and hardware systems that rigorously control experimental parameters. Traditional labs often struggle with protocol variability and less consistent data output, highlighting biofoundries' advantage in maintaining cross-platform standardization and efficient customization.
Reproducibility and Data Management in Both Settings
Biofoundries enhance reproducibility through automated workflows and standardized protocols, minimizing human error prevalent in traditional labs. Data management in biofoundries benefits from integrated digital systems that enable real-time tracking, version control, and streamlined data sharing, contrasting with the often fragmented and manual record-keeping in conventional laboratory environments. These advancements lead to more consistent experimental outcomes and improved transparency, crucial for accelerating synthetic biology research and development.
Skillsets and Workforce Requirements
Biofoundries demand advanced interdisciplinary skillsets combining synthetic biology, automation, and data analytics, contrasting with traditional labs that rely more heavily on manual experimental techniques and specialized expertise in singular biological domains. Workforce requirements in biofoundries emphasize proficiency in computational biology, robotics, and systems engineering to manage high-throughput workflows, while traditional labs prioritize deep knowledge in specific experimental procedures and hands-on bench skills. This shift necessitates continuous upskilling and integration of bioinformatics and engineering disciplines to effectively operate biofoundry platforms.
Innovation Acceleration: Speed from Concept to Prototype
Biofoundries accelerate innovation by automating the design-build-test cycle, enabling rapid prototyping and iterative development that traditional labs cannot match. High-throughput robotics and integrated computational tools reduce the time from concept to prototype from months to weeks. This speed enhances the ability to quickly refine biological systems, driving faster breakthroughs in synthetic biology and biotechnology.
Cost Implications and Resource Utilization
Biofoundries significantly reduce costs through automation and high-throughput processing, enabling rapid prototyping and large-scale experimentation with minimal manual labor. Traditional labs incur higher expenses due to labor-intensive procedures, longer project timelines, and recurring costs for consumables and equipment maintenance. Resource utilization in biofoundries is optimized by integrated digital workflows and robotic systems, maximizing efficiency and minimizing waste compared to the more segmented and manual operations in conventional laboratory settings.
Future Trends: Integrating Biofoundries with Traditional Labs
Biofoundries enhance traditional lab capabilities by automating design-build-test-learn cycles, accelerating synthetic biology workflows with high-throughput robotics and machine learning integration. Future trends emphasize hybrid models where biofoundries act as centralized hubs, providing scalable, reproducible processes that traditional labs can access via cloud-based platforms for collaborative innovation. This integration enables streamlined data sharing, rapid prototyping, and optimized resource utilization, driving breakthroughs in biotechnology and personalized medicine development.
Automated high-throughput screening
Biofoundries enable automated high-throughput screening with greater speed and precision compared to traditional labs, dramatically accelerating synthetic biology research and development.
Synthetic biology workflows
Biofoundries accelerate synthetic biology workflows through automated design-build-test-learn cycles, significantly enhancing throughput and reproducibility compared to traditional labs.
Modular cloning platforms
Modular cloning platforms in biofoundries enable rapid, standardized assembly of genetic parts, significantly enhancing efficiency and scalability compared to traditional labs' manual, time-intensive methods.
Digital laboratory automation
Biofoundries leverage advanced digital laboratory automation to enhance precision, scalability, and reproducibility compared to traditional labs reliant on manual processes.
Standardized genetic parts
Biofoundries accelerate genetic engineering by utilizing standardized genetic parts for high-throughput, automated design and assembly, unlike traditional labs that rely on manual, variable methods.
Design-build-test-learn (DBTL) cycle
Biofoundries accelerate the Design-Build-Test-Learn (DBTL) cycle through automation and high-throughput techniques, outperforming traditional labs in efficiency and scalability of synthetic biology workflows.
Robotic liquid handling
Robotic liquid handling systems in biofoundries enable high-throughput, precise, and automated sample processing, significantly outperforming traditional labs in efficiency and reproducibility.
Laboratory Information Management Systems (LIMS)
Biofoundries leverage advanced Laboratory Information Management Systems (LIMS) with integrated automation and data analytics to enhance scalability and reproducibility, whereas traditional labs rely on more manual, less integrated LIMS solutions that limit throughput and data consistency.
BioCAD/BioCAM integration
Biofoundries enhance synthetic biology by integrating BioCAD and BioCAM tools, enabling automated design-to-manufacturing workflows that significantly outperform traditional labs in speed, accuracy, and scalability.
Scale-up bioprocess optimization
Biofoundries accelerate scale-up bioprocess optimization by integrating automated design-build-test-learn cycles, enabling faster iteration and higher data throughput compared to traditional labs.
Biofoundries vs Traditional labs Infographic
 njnir.com
njnir.com