Single-cell sequencing provides detailed genetic information at the individual cell level, revealing cellular heterogeneity and rare cell populations that bulk sequencing may obscure. Bulk sequencing averages signals across many cells, potentially masking the unique transcriptomic or genomic variations within single cells. Advances in single-cell sequencing technologies enable more precise insights into complex biological systems, improving the understanding of development, disease progression, and cellular function.
Table of Comparison
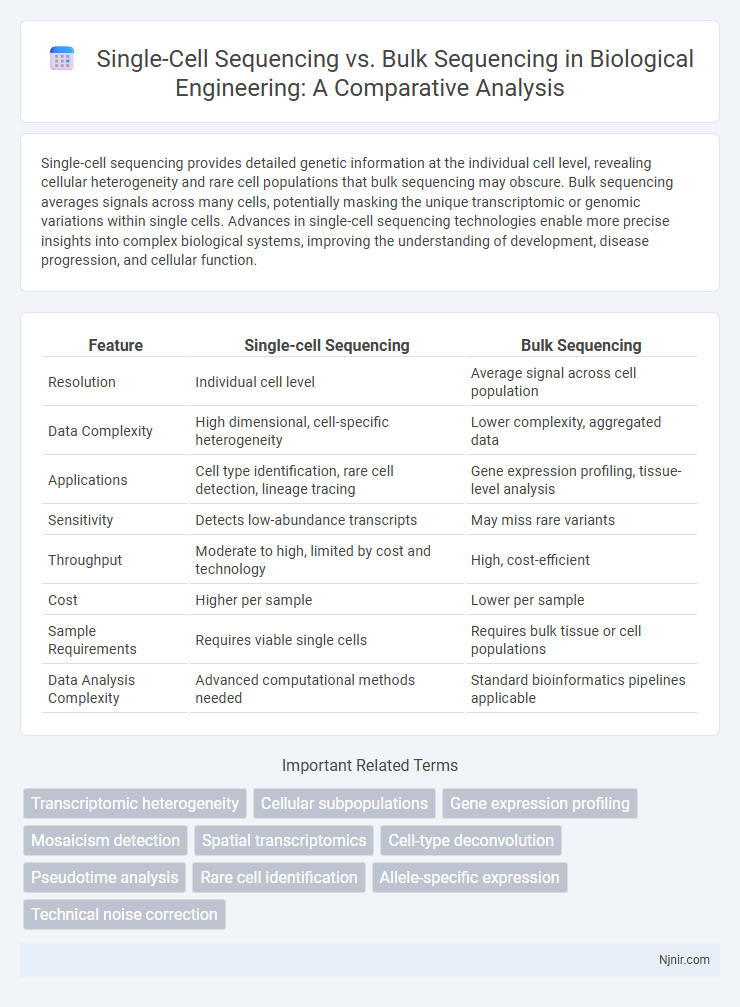
| Feature | Single-cell Sequencing | Bulk Sequencing |
|---|---|---|
| Resolution | Individual cell level | Average signal across cell population |
| Data Complexity | High dimensional, cell-specific heterogeneity | Lower complexity, aggregated data |
| Applications | Cell type identification, rare cell detection, lineage tracing | Gene expression profiling, tissue-level analysis |
| Sensitivity | Detects low-abundance transcripts | May miss rare variants |
| Throughput | Moderate to high, limited by cost and technology | High, cost-efficient |
| Cost | Higher per sample | Lower per sample |
| Sample Requirements | Requires viable single cells | Requires bulk tissue or cell populations |
| Data Analysis Complexity | Advanced computational methods needed | Standard bioinformatics pipelines applicable |
Introduction to Sequencing Techniques in Biological Engineering
Single-cell sequencing enables the analysis of genetic material from individual cells, providing high-resolution insights into cellular heterogeneity and gene expression patterns, essential for understanding complex tissues and disease mechanisms. Bulk sequencing aggregates DNA or RNA from multiple cells, offering an average profile that can mask the diversity present within cell populations. In biological engineering, selecting between single-cell and bulk sequencing depends on the research objective, with single-cell techniques driving advances in personalized medicine and cellular-level biological engineering.
Overview of Single-Cell Sequencing
Single-cell sequencing enables the analysis of genomic, transcriptomic, or epigenomic profiles at the individual cell level, revealing cellular heterogeneity masked in bulk sequencing. This technology captures rare cell populations, dynamic cellular states, and complex tissue architectures, providing high-resolution insights into developmental biology, cancer heterogeneity, and immune responses. Key methods include single-cell RNA sequencing (scRNA-seq), single-cell DNA sequencing, and single-cell ATAC-seq, each critical for understanding gene expression, mutation landscapes, and chromatin accessibility in single cells.
Fundamentals of Bulk Sequencing
Bulk sequencing analyzes pooled DNA or RNA from many cells, providing an averaged genetic or transcriptomic profile that reflects the collective molecular composition. It excels in detecting common mutations, gene expression patterns, and variants in heterogeneous samples but lacks resolution for identifying cell-to-cell variability. The method relies on high-throughput sequencing technologies that aggregate signals from millions of cells, making it cost-effective for population-level genetic insights but limited in dissecting cellular heterogeneity.
Key Technological Differences
Single-cell sequencing enables the analysis of genetic material at the individual cell level, capturing cellular heterogeneity and rare cell populations that bulk sequencing averages out by analyzing mixed cell populations. Key technological differences include the requirement of microfluidic or droplet-based platforms for isolating single cells in single-cell sequencing, whereas bulk sequencing processes pooled DNA/RNA samples without separation. Single-cell sequencing generates sparse, high-dimensional data requiring specialized bioinformatics pipelines, contrasting with the more uniform data output and established analysis workflows of bulk sequencing.
Resolution and Sensitivity Comparison
Single-cell sequencing offers unparalleled resolution by analyzing the genomic or transcriptomic information of individual cells, enabling the detection of cellular heterogeneity and rare cell populations that bulk sequencing averages out. Bulk sequencing aggregates genetic material from thousands to millions of cells, resulting in higher sensitivity for common variants but losing single-cell resolution and masking cell-to-cell variability. The sensitivity of single-cell sequencing is limited by technical noise and dropout events, whereas bulk sequencing provides robust quantification of abundant transcripts but lacks the ability to resolve cellular diversity.
Applications in Biomedical Research
Single-cell sequencing enables the analysis of gene expression and genetic variation at the individual cell level, providing insights into cellular heterogeneity and rare cell populations critical in cancer research and developmental biology. Bulk sequencing aggregates signals from mixed cell populations, offering a comprehensive overview of tissue-level genomic and transcriptomic states ideal for identifying common mutations and overall gene expression profiles. In biomedical research, single-cell sequencing is pivotal for precision medicine and immunology, while bulk sequencing remains essential for large-scale studies and biomarker discovery.
Data Analysis: Challenges and Solutions
Single-cell sequencing presents unique data analysis challenges including high dimensionality, dropout events, and increased noise compared to bulk sequencing, which averages signals across a population of cells. Solutions involve advanced computational methods like dimensionality reduction, imputation algorithms, and clustering techniques designed specifically for single-cell data to accurately identify cell types and states. In contrast, bulk sequencing data analysis relies on established normalization and differential expression tools but lacks the resolution to capture cellular heterogeneity, making single-cell approaches critical for detailed biological insights.
Cost and Throughput Considerations
Single-cell sequencing offers high-resolution insights into cellular heterogeneity but comes with significantly higher costs and lower throughput compared to bulk sequencing, which processes aggregated cell populations more economically and efficiently. Bulk sequencing provides faster data generation and is suitable for large sample volumes, while single-cell sequencing demands advanced instrumentation and increased computational resources, driving up expenses. Cost-effectiveness and throughput capacity are critical factors influencing the choice between single-cell and bulk sequencing methods based on experimental scale and research objectives.
Case Studies in Biological Engineering
Single-cell sequencing reveals cellular heterogeneity by analyzing individual cell genomes or transcriptomes, enabling precise identification of rare cell types and gene expression patterns crucial for biological engineering applications. Bulk sequencing aggregates signals from mixed cell populations, potentially obscuring distinct molecular signatures, which limits resolution in complex tissue studies. Case studies demonstrate single-cell sequencing's superiority in engineering targeted cell therapies, synthetic biology constructs, and regenerative medicine by providing high-resolution data unattainable through bulk sequencing.
Future Perspectives and Emerging Trends
Single-cell sequencing offers unprecedented resolution for analyzing cellular heterogeneity, enabling breakthroughs in personalized medicine and cancer research, while bulk sequencing remains valuable for capturing averaged genomic data across populations. Emerging trends include integrating multi-omics single-cell approaches and leveraging AI-powered analysis pipelines to enhance data interpretation and scalability. Future perspectives highlight advances in spatial transcriptomics and real-time sequencing technologies that promise to revolutionize disease diagnostics and therapeutic strategies.
Transcriptomic heterogeneity
Single-cell sequencing reveals transcriptomic heterogeneity by profiling gene expression at the individual cell level, unlike bulk sequencing which averages signals across mixed cell populations and obscures cellular diversity.
Cellular subpopulations
Single-cell sequencing enables precise analysis of cellular subpopulations by capturing gene expression variability within individual cells, whereas bulk sequencing averages signals across heterogeneous cell populations, obscuring distinct cellular subtypes.
Gene expression profiling
Single-cell sequencing provides high-resolution gene expression profiling by analyzing individual cells, revealing cellular heterogeneity, while bulk sequencing measures average gene expression across mixed cell populations, potentially masking distinct cellular profiles.
Mosaicism detection
Single-cell sequencing offers superior sensitivity in detecting mosaicism compared to bulk sequencing by analyzing genetic variations at the individual cell level, enabling precise identification of rare mosaic mutations.
Spatial transcriptomics
Single-cell sequencing provides high-resolution spatial transcriptomics by analyzing gene expression at the individual cell level, unlike bulk sequencing which averages signals across heterogeneous cell populations, thereby losing spatial and cellular context.
Cell-type deconvolution
Single-cell sequencing enables precise cell-type deconvolution by analyzing individual cellular transcripts, whereas bulk sequencing averages gene expression across mixed cell populations, limiting resolution.
Pseudotime analysis
Single-cell sequencing enables precise pseudotime analysis by capturing individual cell trajectories and dynamic gene expression changes, whereas bulk sequencing averages signals across heterogeneous populations, obscuring temporal cellular progression.
Rare cell identification
Single-cell sequencing enables precise identification of rare cell populations by analyzing individual cellular genomes or transcriptomes, while bulk sequencing averages signals across millions of cells, often masking rare cell-specific variations.
Allele-specific expression
Single-cell sequencing enables precise identification of allele-specific expression patterns within individual cells, unlike bulk sequencing which averages signals across heterogeneous populations and masks cell-to-cell variation.
Technical noise correction
Single-cell sequencing requires advanced technical noise correction methods to accurately distinguish true biological variation from amplification and dropout errors, whereas bulk sequencing averaging reduces noise but masks individual cell heterogeneity.
Single-cell sequencing vs Bulk sequencing Infographic
 njnir.com
njnir.com