Organoid culture replicates the three-dimensional architecture and complex cell interactions of native tissues, providing more physiologically relevant models than traditional 2D cell culture. This 3D environment supports cellular differentiation and function, enabling advanced studies in disease modeling, drug screening, and regenerative medicine. In contrast, 2D cell culture often fails to mimic in vivo conditions, limiting its predictive accuracy for biological processes.
Table of Comparison
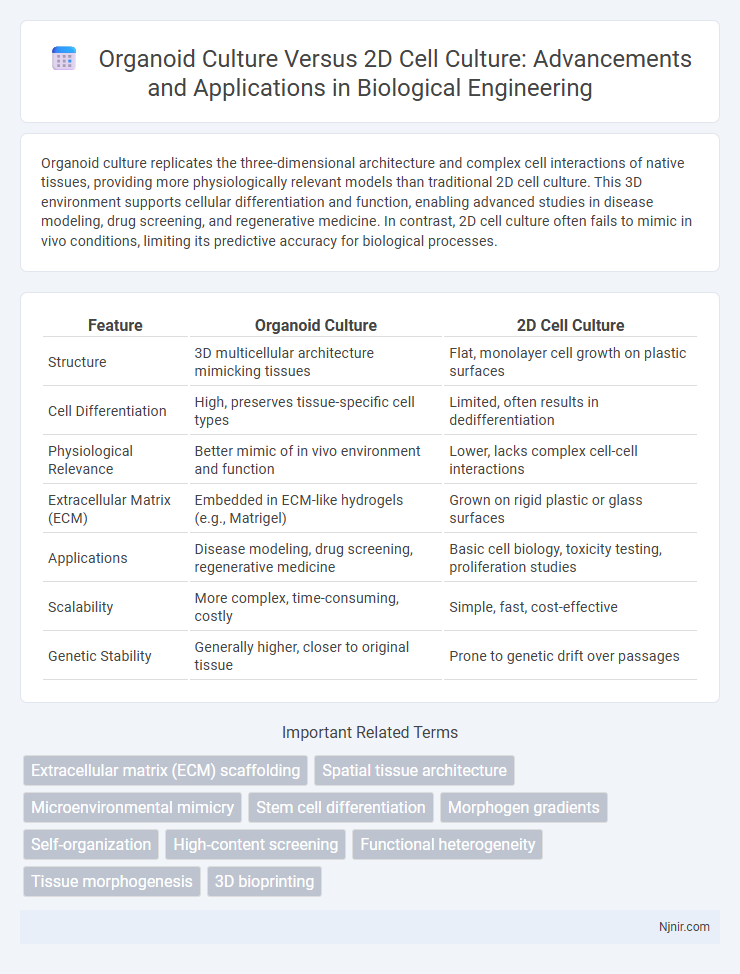
| Feature | Organoid Culture | 2D Cell Culture |
|---|---|---|
| Structure | 3D multicellular architecture mimicking tissues | Flat, monolayer cell growth on plastic surfaces |
| Cell Differentiation | High, preserves tissue-specific cell types | Limited, often results in dedifferentiation |
| Physiological Relevance | Better mimic of in vivo environment and function | Lower, lacks complex cell-cell interactions |
| Extracellular Matrix (ECM) | Embedded in ECM-like hydrogels (e.g., Matrigel) | Grown on rigid plastic or glass surfaces |
| Applications | Disease modeling, drug screening, regenerative medicine | Basic cell biology, toxicity testing, proliferation studies |
| Scalability | More complex, time-consuming, costly | Simple, fast, cost-effective |
| Genetic Stability | Generally higher, closer to original tissue | Prone to genetic drift over passages |
Introduction to Organoid and 2D Cell Cultures
Organoid culture involves three-dimensional cell clusters derived from stem cells that mimic the organization and function of real organs, offering enhanced physiological relevance compared to traditional two-dimensional (2D) cell cultures. 2D cell culture grows cells in a flat monolayer on plastic surfaces, providing simplicity and cost-effectiveness but lacking complex cell interactions and architecture. Organoids better replicate in vivo tissue environments, making them essential for advanced disease modeling, drug screening, and regenerative medicine.
Historical Development of Cell Culture Techniques
Organoid culture emerged as an advanced 3D cell culture technique, building upon the foundational 2D cell culture methods developed in the early 20th century. Pioneering work by Ross Harrison in 1907 established the first in vitro cell culture using 2D monolayers, which dominated biomedical research for decades due to simplicity and ease of observation. The introduction of stem cell biology and enhanced extracellular matrix scaffolds in the early 2000s revolutionized the field, enabling the development of organoid cultures that more accurately recapitulate complex tissue architecture and function.
Structural Differences: 3D Organoids vs 2D Monolayers
Organoid culture mimics the in vivo environment by supporting three-dimensional cellular architecture, enabling complex cell-cell and cell-matrix interactions, while 2D cell culture grows cells in flat monolayers lacking spatial organization. The 3D structure of organoids allows for the development of tissue-specific features such as differentiated cell types and extracellular matrix deposition, which are absent or limited in 2D cultures. These structural differences impact cellular behavior, gene expression, and drug response, making organoids more physiologically relevant for disease modeling and regenerative medicine.
Mimicking In Vivo Physiology: Advantages of Organoid Cultures
Organoid cultures provide a three-dimensional structure that closely mimics the complex architecture and cellular interactions of in vivo tissues, offering more accurate models for studying organ development and disease progression. Unlike 2D cell cultures, organoids exhibit spatial organization, cell differentiation, and microenvironmental cues essential for replicating physiological function. This enhanced mimicry of organ-specific functionality improves the reliability of drug screening and personalized medicine applications.
Limitations of Traditional 2D Cell Culture Models
Traditional 2D cell culture models have limitations in replicating the complex three-dimensional architecture and microenvironment of tissues, leading to altered cell morphology, function, and gene expression. These flat cultures often fail to mimic in vivo interactions, impacting drug response and disease modeling accuracy. Organoid cultures overcome these issues by providing a more physiologically relevant 3D structure that better recapitulates tissue-specific characteristics.
Applications in Disease Modeling and Personalized Medicine
Organoid culture offers a three-dimensional architecture that closely mimics in vivo tissue organization, enhancing the accuracy of disease modeling for conditions like cancer and genetic disorders compared to traditional 2D cell culture. This advanced system enables personalized medicine by allowing patient-derived organoids to be used for drug screening and predicting individual therapeutic responses. Organoids facilitate complex cell-cell interactions and microenvironmental factors, providing superior insights into disease mechanisms and treatment efficacy.
Technical Challenges and Standardization Issues
Organoid culture presents significant technical challenges including reproducibility, complexity in maintaining 3D structures, and variability in extracellular matrix composition, complicating consistent growth and phenotype expression. In contrast, 2D cell culture offers relatively straightforward standardization with uniform cell monolayers but lacks physiological relevance and cell-cell interactions critical for accurate modeling. Standardization issues in organoid culture are exacerbated by batch-to-batch differences in Matrigel and growth factor concentrations, impacting experimental comparability and scalability for drug screening and disease modeling.
Advances in Biomaterials and Culture Systems
Advances in biomaterials have significantly enhanced organoid culture by providing 3D matrices that closely mimic the extracellular matrix, supporting cell differentiation and tissue organization beyond the limitations of 2D cell culture. Innovative hydrogel formulations, such as synthetic PEG-based matrices and naturally derived materials like Matrigel or collagen, enable precise control over mechanical properties and biochemical cues crucial for organoid development. New culture systems integrating microfluidics and bioreactors improve nutrient delivery and waste removal, fostering more physiologically relevant models compared to static 2D monolayers.
Future Perspectives in Biological Engineering
Organoid culture offers a three-dimensional, physiologically relevant model that closely mimics in vivo tissue architecture, surpassing 2D cell culture limitations in cellular differentiation and microenvironmental interactions. Advances in bioengineering techniques, such as microfluidics and biomaterial scaffolds, enable precise control over organoid growth, enhancing their potential for drug screening, disease modeling, and personalized medicine. Integration of organoids with high-throughput imaging and single-cell sequencing technologies is expected to accelerate discoveries in developmental biology and regenerative therapies.
Conclusion: Choosing the Right Model for Research
Organoid culture offers a 3D microenvironment that closely mimics in vivo tissue architecture, providing more physiologically relevant data compared to traditional 2D cell culture. Researchers should select organoid models for studies requiring complex cell-cell interactions and tissue-specific functions, while 2D cultures remain suitable for high-throughput screening and mechanistic assays. The choice ultimately depends on the specific research goals, balancing complexity with experimental scalability and cost.
Extracellular matrix (ECM) scaffolding
Organoid culture utilizes three-dimensional extracellular matrix (ECM) scaffolding to mimic in vivo tissue architecture and cell-cell interactions, unlike 2D cell culture which relies on flat surfaces lacking physiological ECM support.
Spatial tissue architecture
Organoid culture replicates complex spatial tissue architecture in three dimensions, providing a more physiologically relevant environment compared to the flat, two-dimensional arrangement of cells in traditional 2D cell culture.
Microenvironmental mimicry
Organoid culture provides advanced microenvironmental mimicry by replicating 3D tissue architecture and cellular heterogeneity, unlike traditional 2D cell culture which lacks spatial organization and complex cell-cell interactions.
Stem cell differentiation
Organoid culture enhances stem cell differentiation by providing a 3D extracellular matrix that closely mimics in vivo conditions, resulting in more physiologically relevant tissue organization compared to traditional 2D cell culture.
Morphogen gradients
Organoid culture more accurately replicates in vivo morphogen gradients compared to 2D cell culture, enabling more physiologically relevant tissue patterning and cellular differentiation studies.
Self-organization
Organoid culture enables self-organization by mimicking in vivo tissue architecture and cellular interactions, whereas 2D cell culture lacks this complexity and spatial organization.
High-content screening
High-content screening in organoid culture provides more physiologically relevant data and complex cellular interactions compared to traditional 2D cell culture, enhancing drug discovery and toxicity assessment accuracy.
Functional heterogeneity
Organoid culture exhibits superior functional heterogeneity compared to 2D cell culture by closely mimicking the three-dimensional architecture and diverse cell types found in native tissues.
Tissue morphogenesis
Organoid culture more accurately replicates tissue morphogenesis by promoting 3D cellular organization and differentiation compared to traditional 2D cell culture.
3D bioprinting
3D bioprinting enables the creation of complex organoid cultures that closely mimic in vivo tissue architecture and function, surpassing the limitations of traditional 2D cell cultures in drug screening and disease modeling.
Organoid culture vs 2D cell culture Infographic
 njnir.com
njnir.com