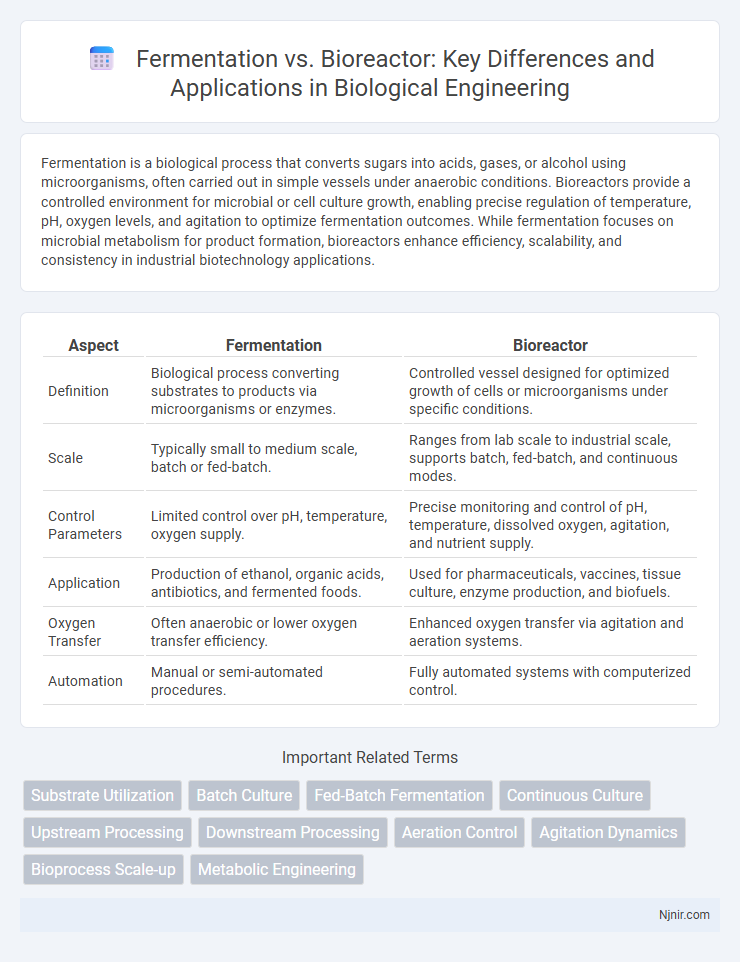
Fermentation is a biological process that converts sugars into acids, gases, or alcohol using microorganisms, often carried out in simple vessels under anaerobic conditions. Bioreactors provide a controlled environment for microbial or cell culture growth, enabling precise regulation of temperature, pH, oxygen levels, and agitation to optimize fermentation outcomes. While fermentation focuses on microbial metabolism for product formation, bioreactors enhance efficiency, scalability, and consistency in industrial biotechnology applications.
Table of Comparison
| Aspect | Fermentation | Bioreactor |
|---|---|---|
| Definition | Biological process converting substrates to products via microorganisms or enzymes. | Controlled vessel designed for optimized growth of cells or microorganisms under specific conditions. |
| Scale | Typically small to medium scale, batch or fed-batch. | Ranges from lab scale to industrial scale, supports batch, fed-batch, and continuous modes. |
| Control Parameters | Limited control over pH, temperature, oxygen supply. | Precise monitoring and control of pH, temperature, dissolved oxygen, agitation, and nutrient supply. |
| Application | Production of ethanol, organic acids, antibiotics, and fermented foods. | Used for pharmaceuticals, vaccines, tissue culture, enzyme production, and biofuels. |
| Oxygen Transfer | Often anaerobic or lower oxygen transfer efficiency. | Enhanced oxygen transfer via agitation and aeration systems. |
| Automation | Manual or semi-automated procedures. | Fully automated systems with computerized control. |
Introduction to Fermentation and Bioreactors
Fermentation is a metabolic process that converts carbohydrates into alcohols or acids using microorganisms under anaerobic conditions, widely applied in food, beverage, and pharmaceutical industries. Bioreactors are controlled vessels designed to optimize the growth and metabolic activity of microorganisms or cells, enabling scalable production of bio-products through precise regulation of parameters like temperature, pH, and oxygen levels. The integration of fermentation processes within bioreactors enhances efficiency, product yield, and consistency, making them essential tools in industrial biotechnology.
Historical Evolution of Fermentation Technologies
The historical evolution of fermentation technologies began with ancient practices of natural fermentation used in food and beverage production, gradually advancing through the Industrial Revolution with the introduction of controlled fermentation processes. The development of bioreactors in the 20th century marked a significant turning point, enabling precise control of environmental parameters to optimize microbial growth and product yield. Modern bioreactors integrate automation, real-time monitoring, and scalable designs, distinguishing them from traditional fermentation vessels and revolutionizing biotechnology and pharmaceutical industries.
Design and Functionality of Bioreactors
Bioreactors are engineered systems designed to provide optimal environmental conditions such as temperature, pH, oxygen supply, and agitation for controlled fermentation processes. Unlike traditional fermentation tanks, bioreactors feature advanced monitoring and control technologies, including sensors and automated feedback loops, to maintain consistent microbial or cell culture growth. Their design incorporates aspects like scale-up capability, aseptic conditions, and efficient nutrient distribution, enhancing product yield and process reproducibility.
Types of Fermentation Processes
Batch, fed-batch, and continuous fermentation represent the primary types of fermentation processes, each offering distinct operational advantages in bioreactor systems. Batch fermentation involves cultivating microbes in a closed system until nutrients deplete, suitable for producing antibiotics and enzymes. Continuous fermentation maintains constant input and output, optimizing productivity for large-scale alcohol and organic acid production, while fed-batch allows controlled nutrient addition to enhance yield and prevent inhibition.
Key Differences Between Fermentation and Bioreactor Systems
Fermentation involves the biological process where microorganisms convert substrates into desired products under controlled conditions, typically in simpler setups like fermentation tanks. Bioreactor systems are advanced vessels designed to provide precise control over physical and chemical parameters such as pH, temperature, oxygen levels, and agitation, optimizing microbial growth and product yield. The key differences lie in the complexity and control capacities, with bioreactors offering enhanced monitoring and scalability compared to traditional fermentation methods.
Applications of Fermentation in Biotechnology
Fermentation plays a crucial role in biotechnology by enabling the production of antibiotics, enzymes, biofuels, and organic acids through the metabolic activity of microorganisms. Unlike bioreactors, which serve as controlled environments for the growth of cells or tissues, fermentation specifically harnesses microbial processes to convert substrates into valuable biochemical products. Industrial applications of fermentation include the manufacturing of pharmaceuticals, food additives, and renewable energy sources, driving innovation in sustainable technologies.
Role of Bioreactors in Industrial-Scale Production
Bioreactors play a crucial role in industrial-scale production by providing controlled environments that optimize fermentation processes, ensuring consistent product yield and quality. They allow precise regulation of temperature, pH, oxygen levels, and nutrient supply, which enhances microbial growth and metabolite production compared to traditional fermentation methods. The scalability and automation capabilities of bioreactors make them indispensable for large-scale manufacturing of pharmaceuticals, biofuels, and food products.
Process Optimization: Fermentation vs. Bioreactors
Process optimization in fermentation emphasizes controlling temperature, pH, and substrate concentration to maximize microbial growth and product yield. In bioreactors, advanced monitoring systems and automated control of parameters such as oxygen transfer rate, agitation speed, and nutrient feed enhance process efficiency and scalability. The integration of real-time data analytics in bioreactors supports precise adjustments, resulting in higher productivity and reduced operational costs compared to traditional fermentation methods.
Challenges and Limitations in Each Approach
Fermentation faces challenges such as contamination risks, limited oxygen transfer rates, and difficulties in maintaining optimal pH and temperature, which can affect microbial growth and product yield. Bioreactors present limitations including high operational costs, complex scale-up processes, and the need for sophisticated control systems to maintain precise environmental conditions. Both approaches struggle with process consistency and scalability, impacting industrial efficiency and product quality.
Future Trends in Fermentation and Bioreactor Technologies
Advancements in fermentation and bioreactor technologies emphasize enhanced precision control, real-time monitoring through AI integration, and scalable modular designs to increase efficiency and product yield. Future trends prioritize sustainable processes utilizing genetically engineered microbes and continuous bioprocessing to reduce waste and energy consumption. Investment in smart bioreactors equipped with sensor networks and automation is expected to revolutionize industrial biotechnology and pharmaceutical manufacturing.
Substrate Utilization
Fermentation utilizes organic substrates through microbial metabolism for product synthesis, while bioreactors optimize substrate utilization by controlling environmental conditions to enhance microbial growth and metabolic efficiency.
Batch Culture
Batch culture fermentation in bioreactors involves cultivating microorganisms in a closed system without adding nutrients during the process, allowing controlled growth and metabolite production until substrate depletion.
Fed-Batch Fermentation
Fed-batch fermentation maximizes microbial growth and product yield by intermittently adding nutrients in a controlled bioreactor environment, optimizing substrate concentration and reducing inhibitory metabolite accumulation compared to traditional batch fermentation.
Continuous Culture
Continuous culture in bioreactors enables precise control over fermentation parameters, optimizing microbial growth and product yield compared to traditional batch fermentation methods.
Upstream Processing
Upstream processing in fermentation involves optimizing microbial growth conditions, whereas bioreactors provide controlled environments for precise regulation of parameters like pH, temperature, and oxygen levels to enhance biomass production.
Downstream Processing
Downstream processing in fermentation involves separating and purifying biological products from microbial cultures, whereas bioreactor downstream processing integrates advanced control systems to optimize product recovery and scalability.
Aeration Control
Aeration control in fermentation involves managing oxygen supply for microbial growth, while bioreactors use advanced systems to precisely regulate aeration, ensuring optimal oxygen transfer rates and enhanced productivity.
Agitation Dynamics
Agitation dynamics in fermentation optimize oxygen transfer and nutrient distribution, enhancing microbial growth and product yield, whereas bioreactors employ controlled agitation systems to precisely regulate shear forces and mixing efficiency for scalable and consistent bioprocessing outcomes.
Bioprocess Scale-up
Bioreactor scale-up optimizes controlled environmental parameters and mixing efficiency to enhance fermentation process productivity from laboratory to industrial bioprocessing.
Metabolic Engineering
Metabolic engineering leverages bioreactors for controlled optimization of fermentation processes to enhance microbial production of desired metabolites.
Fermentation vs Bioreactor Infographic
 njnir.com
njnir.com